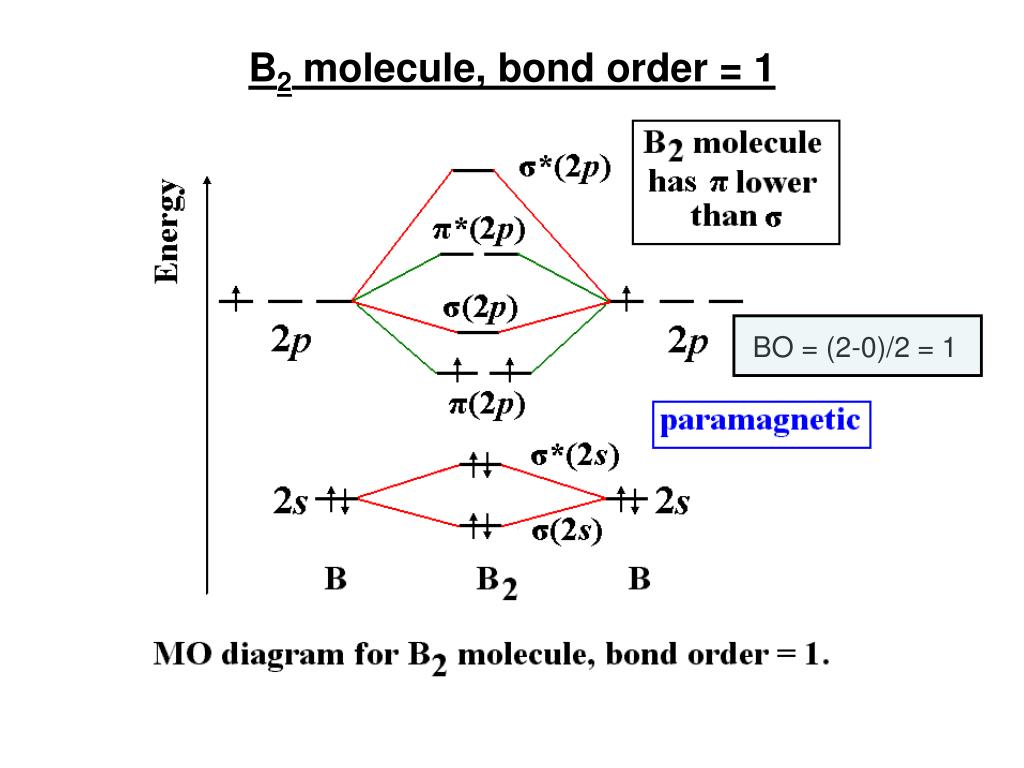
36 b2 molecular orbital diagram bond order
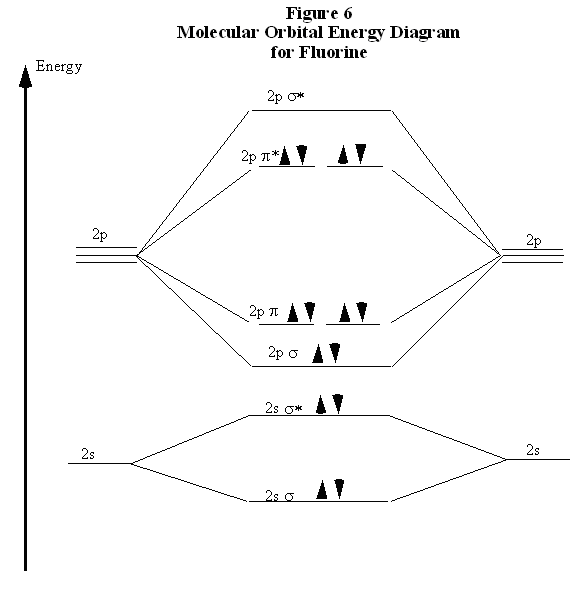
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular… MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine
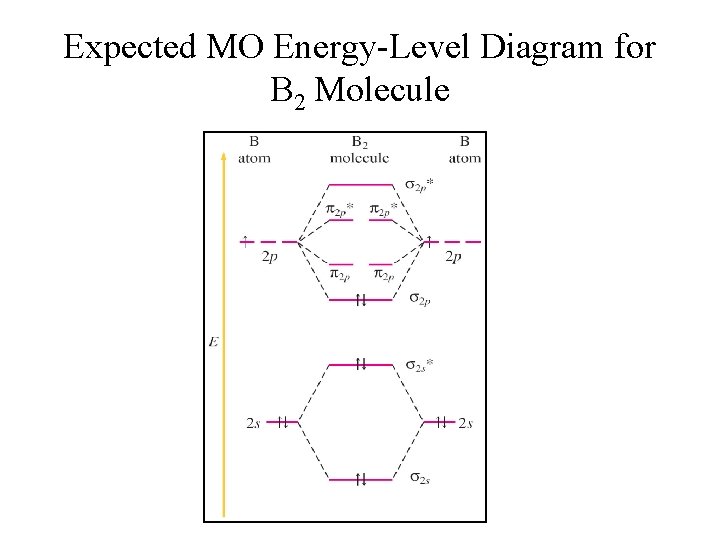
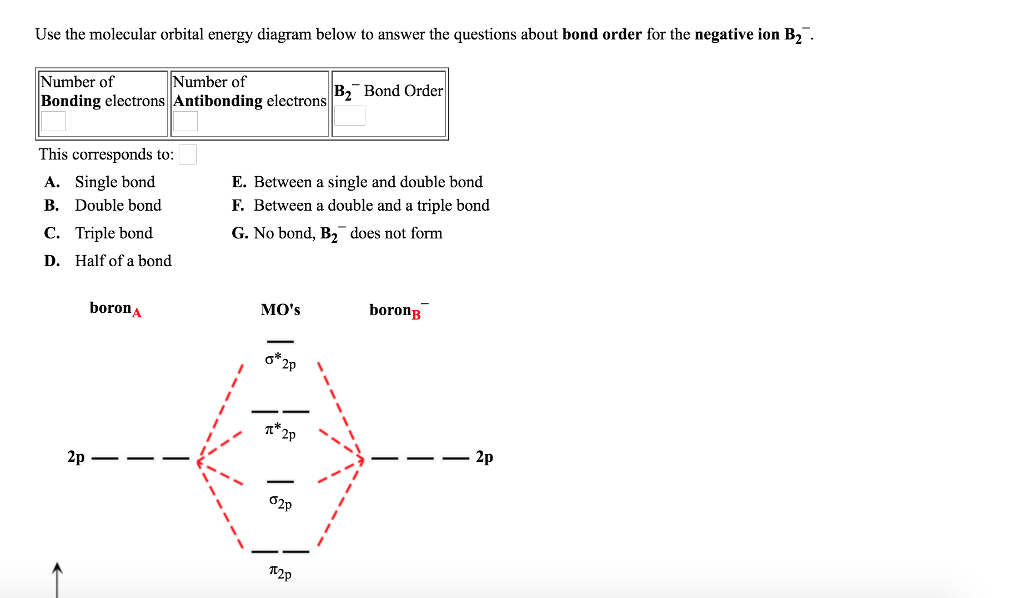
The blank molecular orbital diagram shown here (Figure 1) applies to the valence of diatomic lithium, beryllium, boron, carbon, or nitrogen. Bonding orbitals are marked with sigma or pi and antibonding orbitals with sigma* or pi*. What is the bond order of B2- ?

B2 molecular orbital diagram bond order
Hint: We know that molecular orbital diagrams are used to determine the bonding in a diatomic molecule. The molecular orbital diagrams are used to predict the magnetic properties of a molecule. Molecular orbital diagrams help in determining the bond order of the molecule. Molecular Orbital Theory allows us to predict the distribution of electrons within a molecule. This allows us to predict properties such as bond order, magnetism, and shape.. There are two types of MO diagrams:. Recall that the bonding MOs are those without an asterisk (e.g., σ 1s), while the antibonding MOs are those with an asterisk (e.g., σ 1s *). 14:24This video shows the end of the Be2 molecule MO diagram and explains pi orbitals, paramagnetism, and the ...26 Mar 2014 · Uploaded by Diego Troya
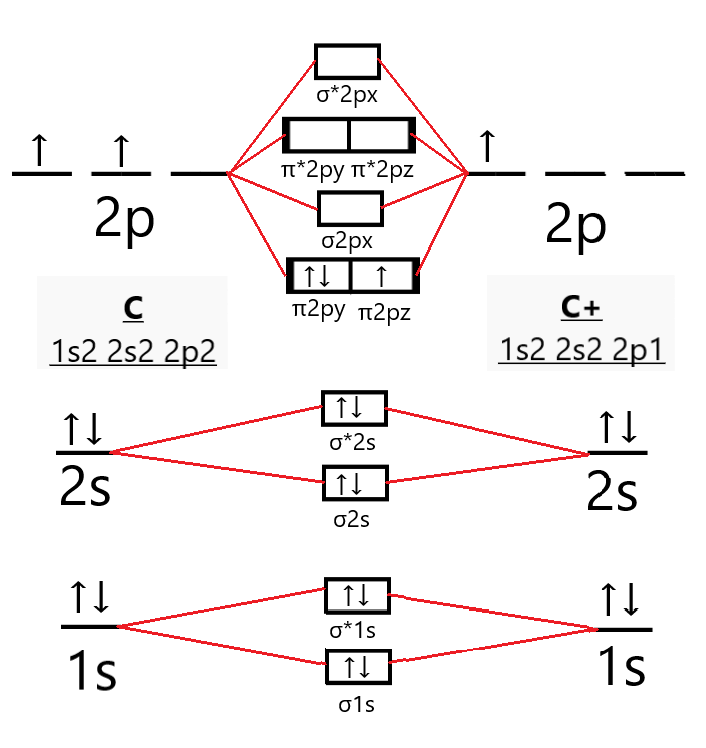
B2 molecular orbital diagram bond order. 6:25This video discusses how to draw the molecular orbital (MO) diagram for the B2(+) molecule. The bond ...4 Jun 2021 · Uploaded by Principia This video discusses how to draw the molecular orbital (MO) diagram for the B2(-) molecule. The bond order of the boron anion is also calculated and the mean... MO electronic configuration:Bond order: Here Nb = 4, Na = 2Bond order = The two boron atom is B2 molecules are linked by one covalent bond. Rating: 4,4 · 740 votes · Free · Android · Educational We’re being asked to determine the bond order of B2- and determine if it is paramagnetic or diamagnetic. For this, we need to determine the bond order for each species. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion.
6:08This video discusses how to draw the molecular orbital (MO) diagram for the B2 (boron) molecule. The bond ...30 May 2021 · Uploaded by Principia 7:14The bond order of B2, C2, and N2 are 1, 2, and 3, respectively. B2 has two unpaired electrons with the ...20 Jun 2019 · Uploaded by Physical Chemistry Tutorial Bond order indicates the stability of a bond. In a more advanced context, bond order does not need to be an integer. Bond Order in Molecular Orbital Theory. In molecular orbital theory, bond order is also defined as the difference, divided by two, between the number of bonding and antibonding electrons; this often, but not always, yields the ... Bond order of B2 molecule. So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = .5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond.
Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ... 14:24This video shows the end of the Be2 molecule MO diagram and explains pi orbitals, paramagnetism, and the ...26 Mar 2014 · Uploaded by Diego Troya Molecular Orbital Theory allows us to predict the distribution of electrons within a molecule. This allows us to predict properties such as bond order, magnetism, and shape.. There are two types of MO diagrams:. Recall that the bonding MOs are those without an asterisk (e.g., σ 1s), while the antibonding MOs are those with an asterisk (e.g., σ 1s *). Hint: We know that molecular orbital diagrams are used to determine the bonding in a diatomic molecule. The molecular orbital diagrams are used to predict the magnetic properties of a molecule. Molecular orbital diagrams help in determining the bond order of the molecule.

Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11

Use Molecular Orbital Theory To Determine Whether He2 2 Or He2 Is More Stable Draw The Molecular Orbital Diagram For Each And Explain Study Com

Use The Molecular Orbital Energy Level Diagram To Show That N 2 Would Be Expected To Have A Triple Bond F 2 A Single Bond And Ne 2 No Bond
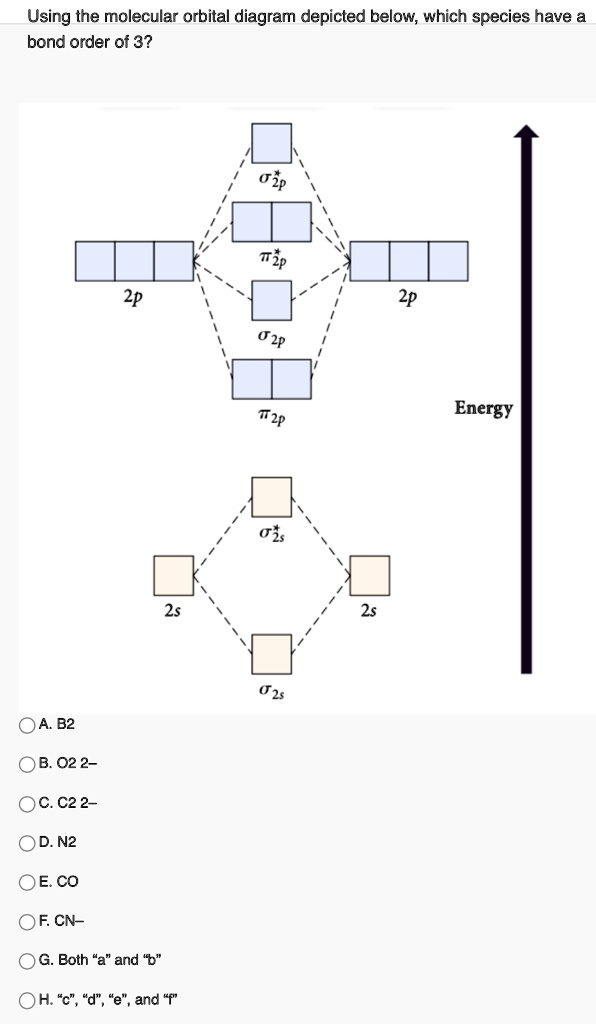
Solved Using The Molecular Orbital Diagram Depicted Below Which Species Have Bond Order Of 3 2p 2p 02p 72p Energy 2s Oa B2 B 02 2 C C22 D N2 Oeco Of Cn G

Calculate The Bond Orders For Li2 And Be2 Molecules Using The Molecular Orbital Diagrams Given In Fig Brainly In

Write Molecular Orbital Configuration Of C2 Predict Magnetic Behaviour And Calculate Its Bond Order H7ch14qq Chemistry Topperlearning Com

Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic N22 B22 Homeworklib
















0 Response to "36 b2 molecular orbital diagram bond order"
Post a Comment