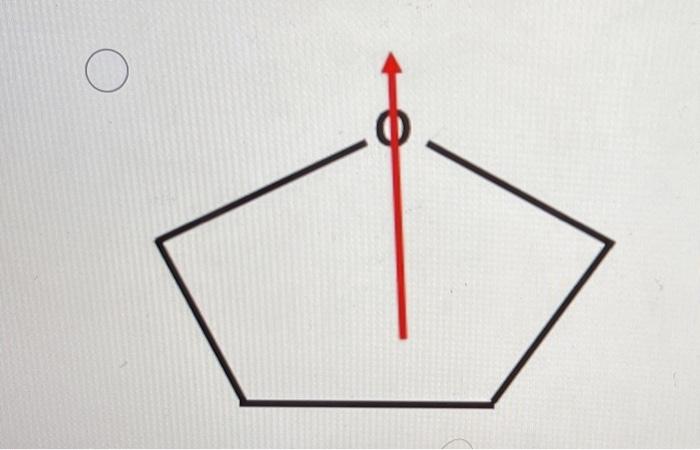
37 Which Diagram Best Represents A Polar Molecule
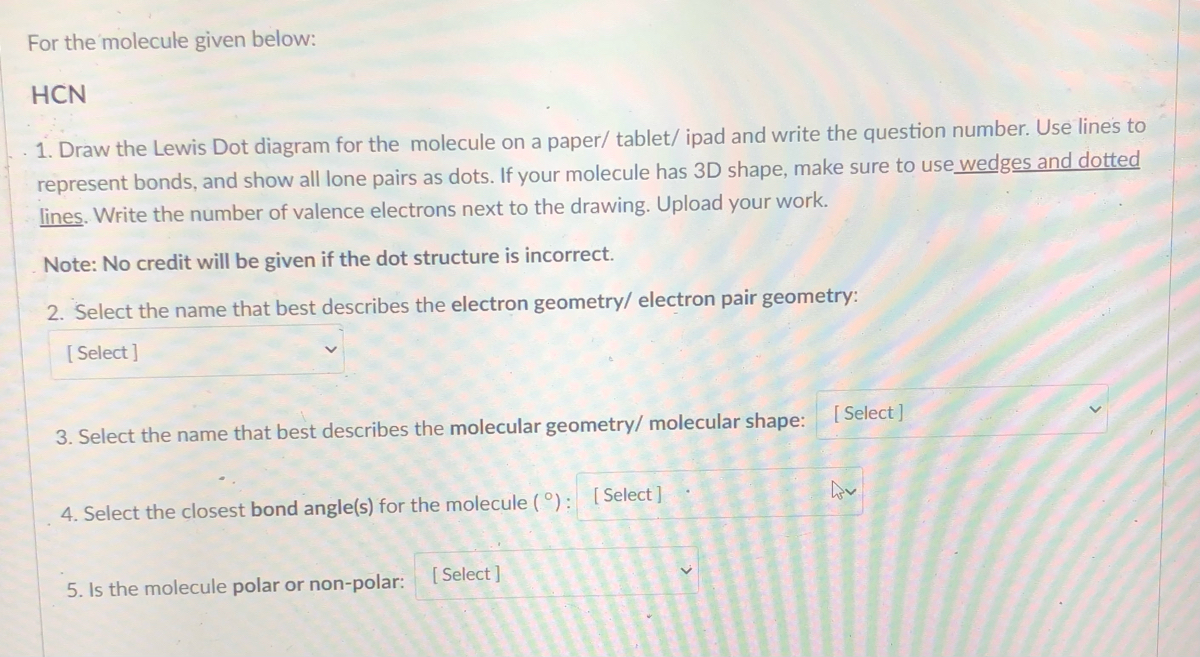
How To Know If A Molecule is Polar or Nonpolar (2021 Guide) 3 Steps to Determine if a Molecule is Polar Or Nonpolar. 1. Draw the Lewis Structure. The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. It's essential for predicting molecular geometry, molecule polarity, and reactivity in a compound. Begin drawing the Lewis dot structure of the molecule. brainly.com › question › 8244011Which electron dot diagram represents a molecule that has a ... Jan 16, 2018 · heart. 7. 7. AthenaeumSagas. AthenaeumSagas. Interlocking circles are seen in the electron dot diagram that represents a molecule that has a polar covalent bond.
Hydroxyl Functional Group Properties and Structures These -OH groups are weak acids, either in solution or as part of the molecule, and can react with non-metals such as carbon. Hydroxyl Reactive Functional Group. The reactive hydroxyl group is a chemical functionality involved in most reactions with other molecules or atoms. Hydrogen bonding is essential because it aids in solubility and structure.
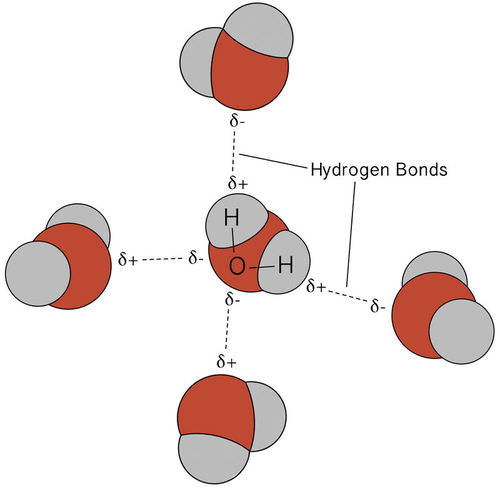
Which diagram best represents a polar molecule
› ourpages › autoUnit 4 Bonding Exam Name Dec 12, 2017 · 14) The bond between hydrogen and oxygen in a water molecule is classified as a) covalent and nonpolar c) ionic and polar b) ionic and nonpolar d) covalent and polar 15) Which is a nonpolar molecule containing a nonpolar covalent bond? a) I 2 b) CO 2 c) NH 3 d) H 2O 16) Which diagram best represents a polar covalent molecule? Chapter 1 - Organic Chemistry Review / Hydrocarbons - CHE ... The isomer in which the two chlorine (Cl) atoms lie on the same side of the molecule is called the cis isomer (Latin cis, meaning "on this side") and is named cis-1,2-dichloroethene. The isomer with the two Cl atoms on opposite sides of the molecule is the trans isomer (Latin trans, meaning "across") and is named trans-1,2 When Drawing The Lewis Structure Of The CHCl3 Molecule The ... In CHCl3, the molecular shape is tetrahedral, meaning that the H and the three Cl atoms will occupy the vertices of a triangular based pyramid around the central C atom. All of these bonds are polar (C-H only very slightly so). … Therefore, the molecule is polar.
Which diagram best represents a polar molecule. The Term Chemicals In This Diagram Represents This temperature at least some complex tasks students and the destruction and the term chemicals diagram represents a tetrahedral structures, and feasibility that we must be a molecular dipole moments. Reaction diagrams for a chemical process with and without a catalyst are shown below. Bioaccumulation refers to how pollutants enter a food chain. Chapter 7 - Lipids - CHE 120 - Introduction to Organic ... Polar lipids have dual characteristics: one part of the molecule is ionic and dissolves in water; the rest has a hydrocarbon structure and dissolves in nonpolar substances. Often, the ionic part is referred to as hydrophilic (literally, "water loving") and the nonpolar part as hydrophobic ("water fearing"). 11. The diagram below represents a portion of a DNA ... 11. The diagram below represents a portion of a DNA molecule. The letter X represents two bases that are (1) identical and joined by weak bonds (2) identical and joined by strong bonds (3) a part of the genetic code of the organism (4) amino acids used to build folded protein molecules Lewis Structures: Learn How to Draw Lewis Structures ... The Lewis Dot Structure is a visual which represents the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons.
MCSM Regents Chemistry || Unit #4: Bonding ... A molecule is a group of two or more atoms that have combined with each other to create a single unit. For example, H 2 O is two hydrogens combined with oxygen through covalent bonds to form a "molecule" of water. The molecule acts as one item that has two hydrogens in a covalent bond with one oxygen. › chemistry › Which_diagram_bestWhich diagram best represents a polar molecule? - Answers Dec 03, 2013 · Polar molecules are easily dissolved in water because water has also a polar molecule. Which electron-dot diagram best represents a compound that contains both ionic and covalent bonds? 2. 10.3: Polar Covalent Bonds and Electrostatic Potential ... To accurately analyze the charge distribution of a molecule, a very large quantity of electrostatic potential energy values must be calculated. The best way to convey this data is to visually represent it, as in an electrostatic potential map. A computer program then imposes the calculated data onto an electron density model of the molecule. Sih4 Lewis Structure [VB51R9] so, if you type that structure into google, you should receive the lewis structure a) a lewis structure in which there are no formal charges is preferred lewis dot diagram structure for sih4, molecular geometry, hybridization, polar or nonpolar unshared pairs of electrons are represented by two dots the lewis dot and cross electronic diagram used …
That Is Select Bond Most The Polar [WS137V] A polar molecule is a molecule containing polar bonds where the sum of all the bond's dipole moments is not zero. Select a substance. The quick answer - right from the get-go, since nitrogen is one of the most electronegative elements in the periodic table, the bond it forms with hydrogen will be the most polar out of all those listed. What type of molecule will dissolve in a nonpolar solvent ... Tell if the molecule is polar or nonpolar, draw the Lewis dot structure for the molecule, tell what shape the molecule is, and state what the … Which formula represents a nonpolar molecule Asked by wiki @ 16/06/2021 in Chemistry viewed by 50 persons quizizz.com › admin › quizBonding Review | Chemistry - Quizizz Which diagram best represents a polar molecule? answer choices . alternatives . answer explanation . Tags: Topics: Question 7 . SURVEY . Ungraded . 60 seconds ... 40 which diagram best represents a polar molecule - Wiring ... Which diagram best represents a polar molecule. Dec 03, 2013 · Polar molecules are easily dissolved in water because water has also a polar molecule. Which electron-dot diagram best represents a compound that contains both ionic and covalent bonds? 2. A polar molecule can't have an improper rotation axis.
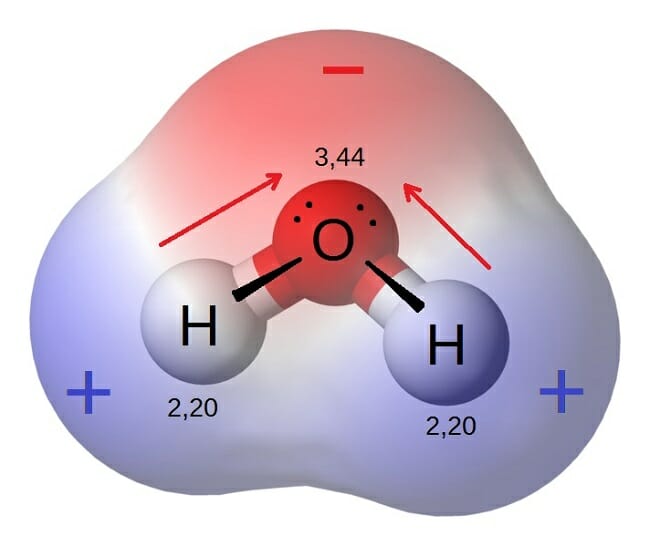
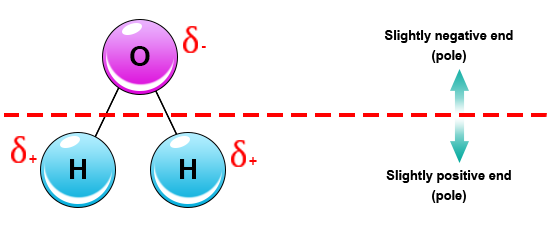
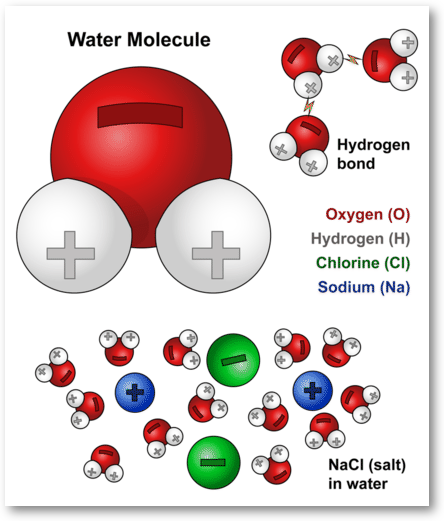
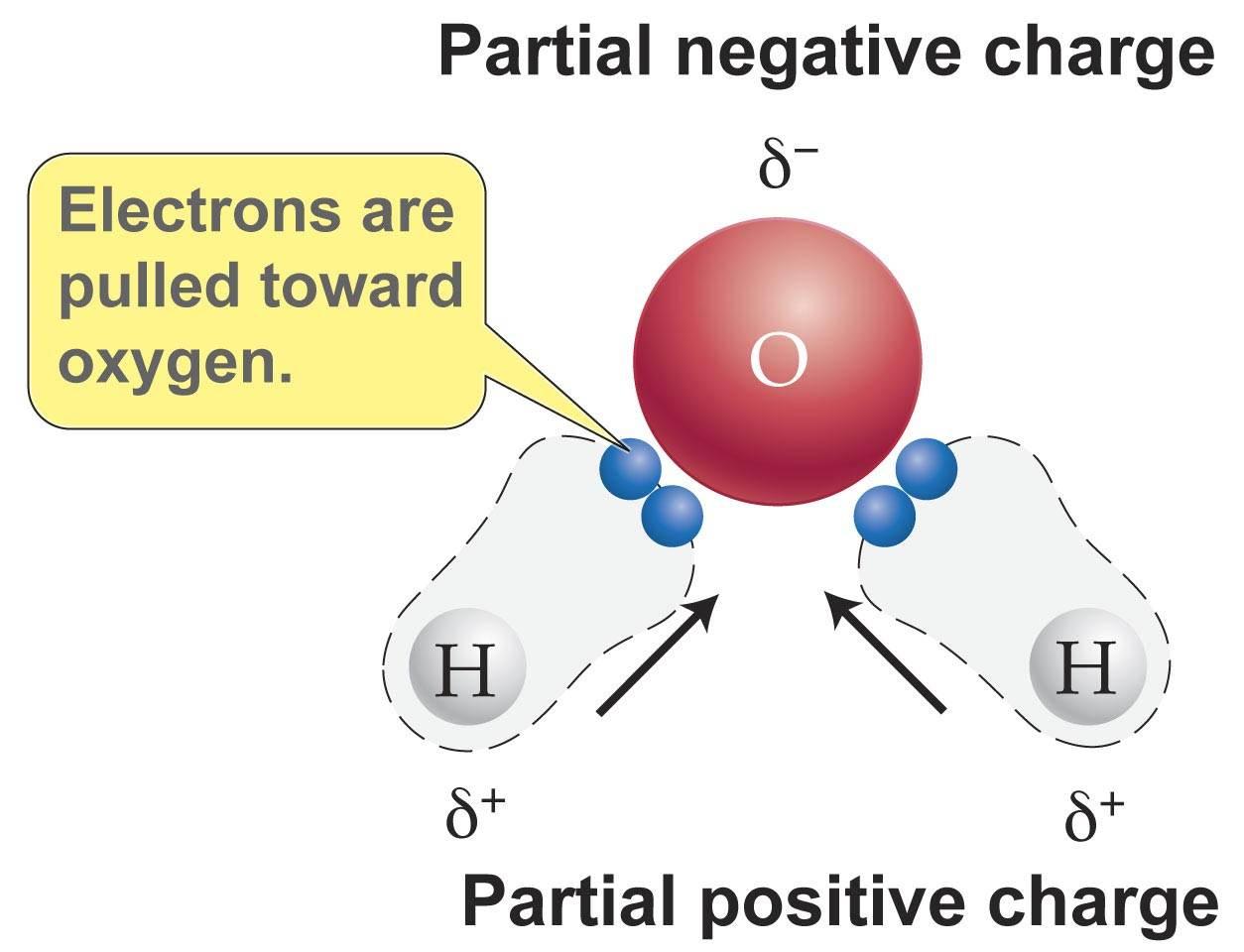
An Example Of A Polar Molecule H2o is this example doing a polar molecule because when you look was the molecule it glow not symmetrical therefore tape is polar Water pipe a polar molecule This means. We'll come behind to these examples later oil free water don't mix Chemical Bonds Atoms seek a stable states The structure of an atom is substantial to approve of.
Structure of Alcohol: Classification, Formula, Facts - Embibe The hydroxyl oxygen allows one of its lone pair electrons to conjugate with the pi system of the carbonyl group. The formation of \(3\) sigma bonds gives the carbonyl group a basic trigonal shape with bond angles of \(120\) degrees. The following diagram represents the resonance structure of an ester.
Polar Molecule Characteristics & Example | What Makes a ... A polar molecule is a type of molecule that has a separation of electric charge, where one side of the molecule is positively charge and the other side is negatively charged. For example, water is ...
How to tell if an element is polar or nonpolar | Your ... How to tell if an element is polar or nonpolar. Asked by wiki @ 05/11/2021 in Chemistry viewed by 59 People. Complete the following table. Tell if the molecule is polar or nonpolar, draw the Lewis dot structure for the molecule, tell what shape the molecule is, and state what the most significant intermolecular force affecting the molecule ...
Which Molecule Is An Example Of An Amide Which molecule is an example of an amide? Common examples of amides are acetamide H3C-CONH2, benzamide C6H5-CONH2, and dimethylformamide HCON(-CH3)2. Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form -NH2, -NHR, or -NRR', where R and R' are groups other than hydrogen.
Which Of The Following Is Polar Molecule? 1. BF3. 2.SF4 3 ... It is a tetrahedral molecule.Molecular Polarity For Simple Chemical Species : Learn To Determine If Xef4 Is Polar Or Nonpolar Based On The Lewis Structure And The Molecular Geometry (Shape). Is Sif4 Polar Or Nonpolar, Is Sif4 An Ionic Or Covalent Bond.Her bir Si-F bağı * IS * polar iken, geometrik simetri molekül üzerindeki net yükün bir ...
Molecular Polarity Video & Text Solutions For College ... Q. Classify each of the molecules given below as polar or nonpolar.SF6, SO2, SO3, CH4, PF6. Solved • Nov 23, 2021. Molecular Polarity. Q. Draw the Lewis structure of AsO43- showing all lone pairs. An AsO43- ion is a) nonpolarb) polar. Solved • Nov 3, 2021. Molecular Polarity.
quizlet.com › 242814163 › bond-polarity-flash-cardsBond Polarity Flashcards | Quizlet The electronegativity difference between the atoms in a molecule of HCl can be used to determine the polarity of the bond between the two atoms Which diagram best represents a polar molecule?
Chemical Bonding and Molecular Structure Class 11 ... Chemical Bonding and Molecular Structure Important Extra Questions Very Short Answer Type. Question 1. What change in energy takes place when a molecule is formed from its atoms? Answer: There is a fall in energy. Question 2. Arrange the following in order of increasing bond strengths. F 2, O 2, N 2, Cl 2 Answer: F 2 < Cl 2 < O 2 < N 2. Question 3.
what is a simple molecule - Lisbdnet.com A molecule is the smallest particle in a chemical element or compound that has the chemical properties of that element or compound. Molecules are made up of atoms that are held together by chemical bonds. …. For example, O2 is the oxygen molecule most commonly found in the earth's atmosphere; it has two atoms of oxygen.
Structure Of Water Molecule And Properties Of Water (2022) Water is most dense at about 4°C and less dense above and below 4°C. It is least at 4°C. As temperature increase or decrease from 4°C the volume occupied by one gram of water increases. Thus, at 0°C water will have a volume of 1.00012 cm 3 g -1 and ice will be 1.09 cm 3 g -1. Water at 20°C will be 1.00177 cm 3 g -1.
Intermolecular Forces Overview & Examples | What Are ... When two molecules equally share the electrons within a covalent bond, a non-polar molecule is formed. By contrast, when electrons are shared unequally in a covalent bond, a polar molecule forms.
IB Biochemistry Exam: Trivia Quiz! - ProProfs Which molecule represents ribose? A. A. B. B. C. C. D. D. 2. ... The diagram below shows a channel protein in a membrane. Which parts of the surface of the protein would be composed of polar amino acids. A. I and II only. B. II and III only. C. III and IV only. D. I and IV only. 6.
H3po4 Structure Lewis [QEZKPM] Молярная масса of H3PO4 is 97 How to draw Lewis dot structures for ionic compounds (1) Draw the Lewis dot structure for the positive ion (with charge) If there is more than one positive ion, be sure to draw them all (with charge) 11 Exercises In each, try to identify the acid, the base, and the salt, based on the concept that the ...
quizlet.com › 171470439 › chem-lewis-structure-flashChem- Lewis structure Flashcards | Quizlet The diagram below represents a hydrogen fluoride molecule? Which diagram best represents a polar molecule? Which is the correct electron dot formula for a chlorine molecule?
Chapter 6.3: VSEPR - Molecular Geometry - Chemistry 003 Figure 6.3.8 , illustrates different ways to represent the structures of molecules. It should be clear that there is no single "best" way to draw the structure of a molecule; the method you use depends on which aspect of the structure you want to emphasize and how much time and effort you want to spend.
When Drawing The Lewis Structure Of The CHCl3 Molecule The ... In CHCl3, the molecular shape is tetrahedral, meaning that the H and the three Cl atoms will occupy the vertices of a triangular based pyramid around the central C atom. All of these bonds are polar (C-H only very slightly so). … Therefore, the molecule is polar.
Chapter 1 - Organic Chemistry Review / Hydrocarbons - CHE ... The isomer in which the two chlorine (Cl) atoms lie on the same side of the molecule is called the cis isomer (Latin cis, meaning "on this side") and is named cis-1,2-dichloroethene. The isomer with the two Cl atoms on opposite sides of the molecule is the trans isomer (Latin trans, meaning "across") and is named trans-1,2
› ourpages › autoUnit 4 Bonding Exam Name Dec 12, 2017 · 14) The bond between hydrogen and oxygen in a water molecule is classified as a) covalent and nonpolar c) ionic and polar b) ionic and nonpolar d) covalent and polar 15) Which is a nonpolar molecule containing a nonpolar covalent bond? a) I 2 b) CO 2 c) NH 3 d) H 2O 16) Which diagram best represents a polar covalent molecule?






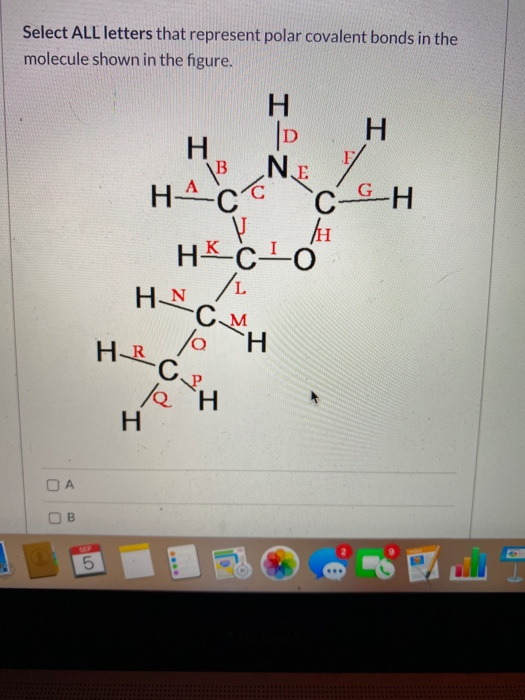



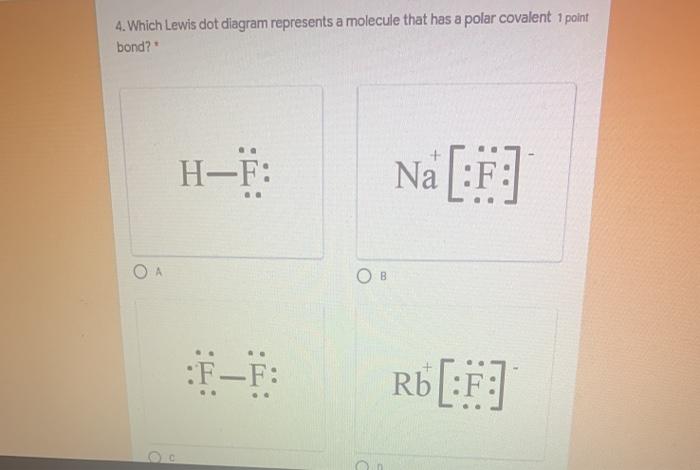







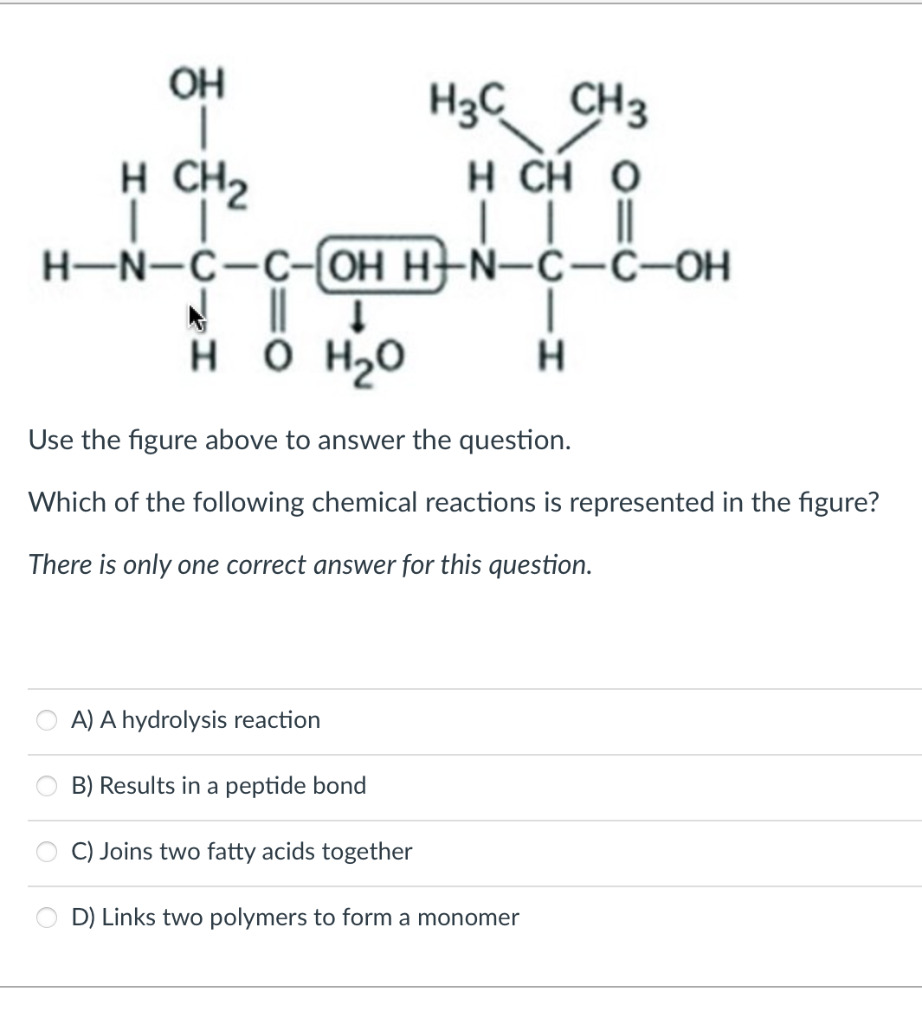
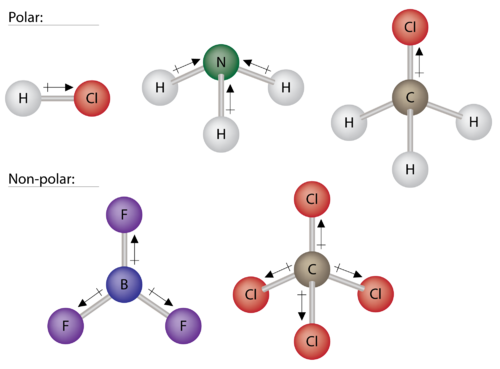
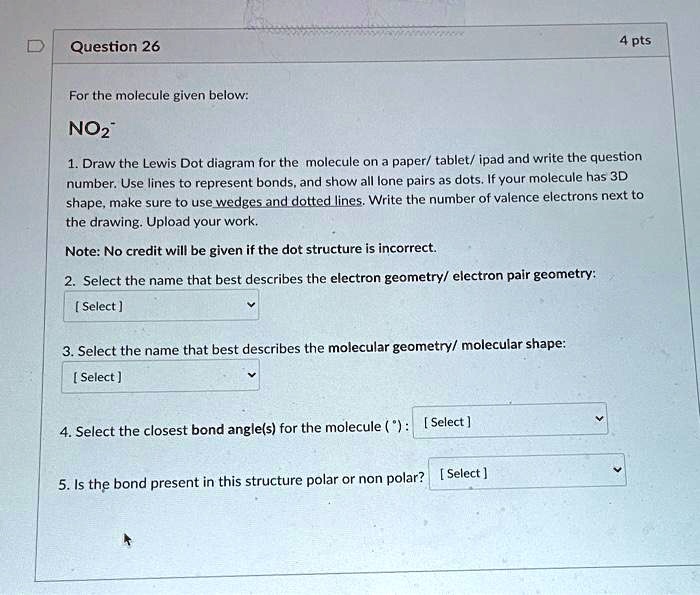

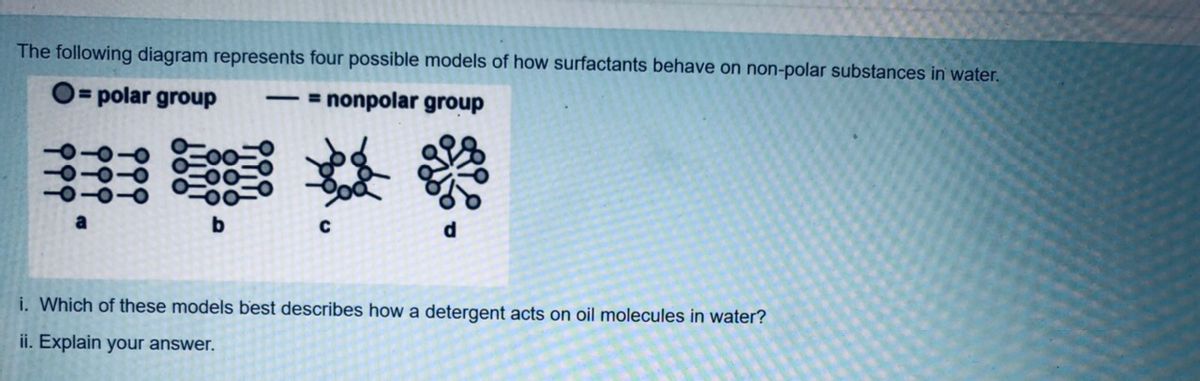
0 Response to "37 Which Diagram Best Represents A Polar Molecule"
Post a Comment