35 how to draw a bohr diagram
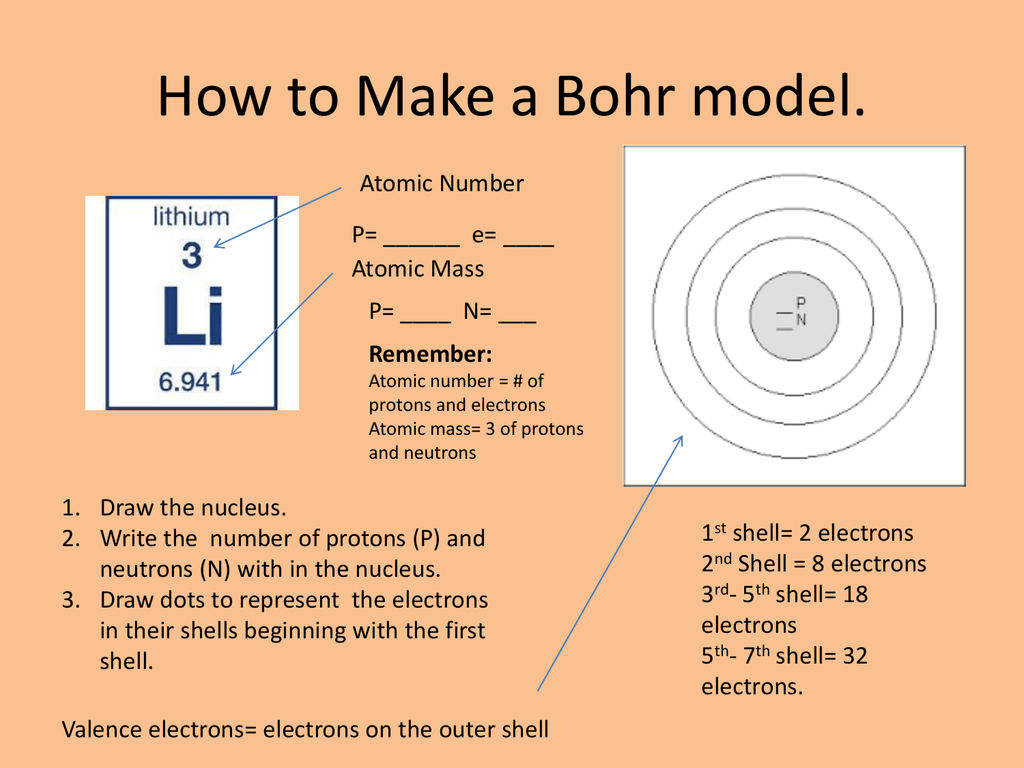
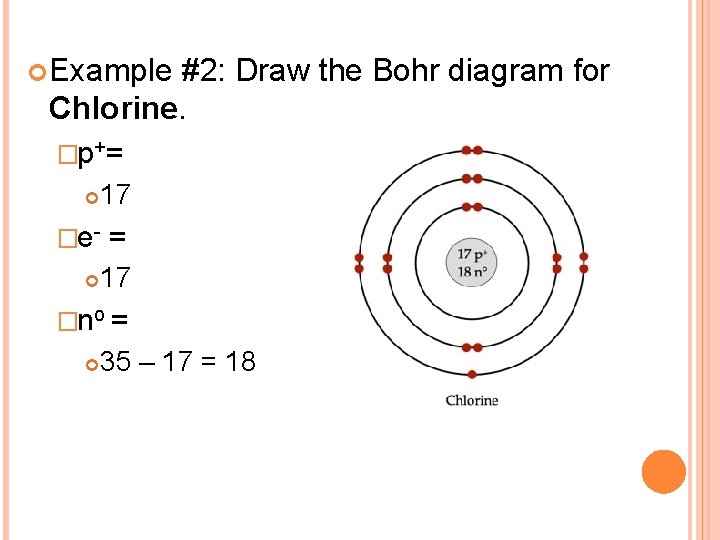
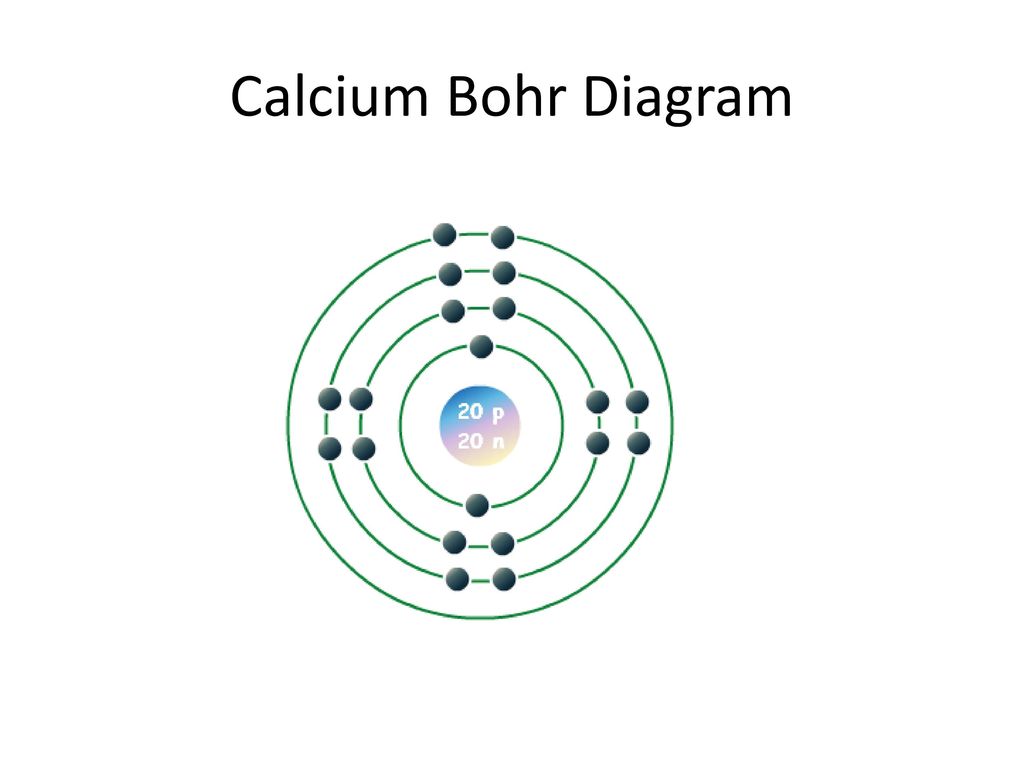
How to draw the Bohr-Rutherford Diagram for Potassium. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on... Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Calcium atom with some simple steps. Steps to draw the Bohr Model of Calcium atom. 1. Find the number of protons, electrons, and neutrons in the Calcium
We certainly tried to draw other groups into the program, but the problem was that to run or contribute to the development of a complex global climate model system, you have to be willing to get your hands dirty, you really have to become involved. You cannot just sit around and have some clever ideas. You cannot work on a complex model some 500 kilometers away. The people we collaborated with ...

How to draw a bohr diagram
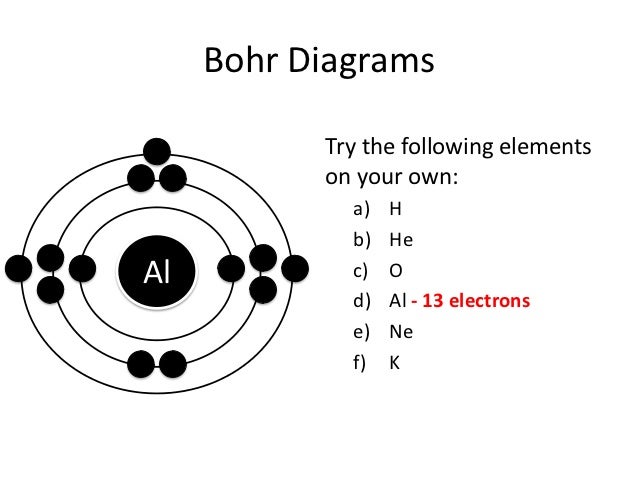
Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. This is because protons and neutrons ... These are what make up the Periodic Table of Elements: This is how a Bohr-Rutherford Diagram look like and this is Nitrogen: 1) There are 10 electrons in Neon. This is how you Calculate Protons, Neutrons, and Electrons in an Atom: This is how you draw the Bohr-Rutherford Diagram: 1) There are 19 electrons in Potassium. The Bohr model works well for very simple atoms such as hydrogen (which has 1 electron) but not for more complex atoms. bohr rutherford diagram drawing task cards exit cards - 28 images - grade stuff on middle school science, ppt differentiated technology day best images about chemistry on activities, watches on, how to draw a bohr rutherford ...
How to draw a bohr diagram. Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Fluorine atom with some simple steps. Steps to draw the Bohr Model of Fluorine atom. 1. Find the number of protons, electrons, and neutrons in the Fluorine atom Bohr Diagrams and Lewis Dot Structures What you've already learned in class and from readings You learned that Electrons can exist in different energy levels You learned that the # of Electrons in an atom are equal to the # of Protons in an atom You learned that the # of Valence Electrons are the outermost Electrons of an Atom What You're about to learn How to draw the Electrons around an ... Once you have found this information, follow the directions to draw your model. 6. C. Carbon. 12.011. 6. 6. 6. 2. 4. Protons: _____ ... HOW TO DRAW BOHR DIAGRAMS - In this video, I'll teach you how to draw bohr diagrams for carbon (C), sodium (Na), and phosphorous (P). The steps to drawing bo...
The Bohr Model.draw a Bohr-Rutherford diagram for nitrogen. draw a Bohr-Rutherford diagram for oxygen. draw a Bohr-Rutherford diagram for fluorine. a Bohr-Rutherford diagram is used to show the numbers and locations of protons, neutrons, and electrons in an atom. step 1. 5 Jul 2016 — I updated the Google Slides and worksheet for my lesson on drawing Bohr Diagrams. This lesson will walk your students through the basics on ... It has only 1 electron, draw it on the right side. Bohr Diagrams. He. Bohr Diagrams. He. Helium is in the 1st period, draw 1 shell. The 1st electron is on the right. The 2nd electron is on the left. Bohr Diagrams. O. Bohr Diagrams. O. Oxygen is in the 2nd period and has 2 shells. Bohr Diagrams 1) Draw a nucleus with the element symbol inside. 2) Carbon is in the 2nd C period, so it has two energy levels, or shells. 3) Draw the shells around the nucleus. 8. Bohr Diagrams 1) Add the electrons. 2) Carbon has 6 electrons. C 3) The first shell can only hold 2 electrons. 9.
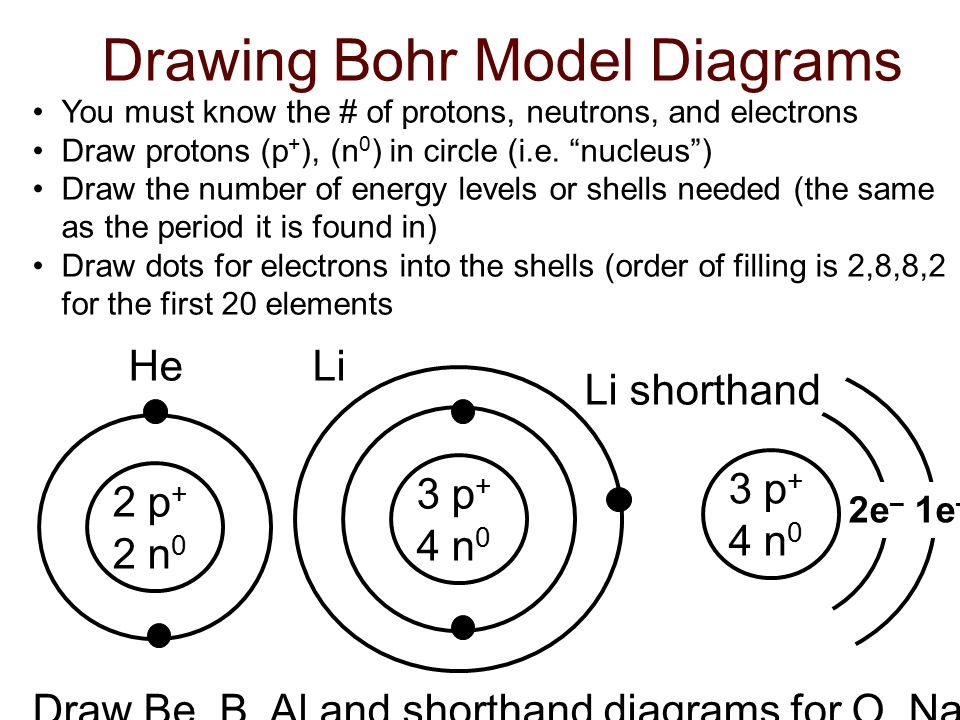
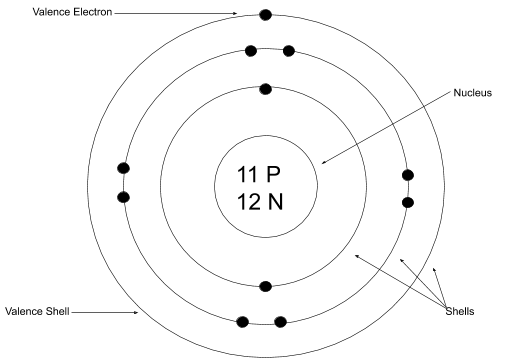
Using the Bohr model, determine the energy, ... Draw the orbital diagram for the valence shell of each of the following atoms: (a) C (b) P (c) V (d) Sb (e) Ru. 53. Use an orbital diagram to describe the electron configuration of the valence shell of each of the following atoms: (a) N (b) Si (c) Fe (d) Te (e) Mo . 54. Using complete subshell notation (1s 2 2s 2 2p 6, and so forth), predict the ... Bohr Diagram. Be. Bohr Diagram. Cl. Bohr Diagram Physical Science Name/Per/Due date_____ Valence Electron Practice. Directions: Give the total number of electrons and the number of valence electrons for each element listed below. Hydrogen 2. Lithium. Beryllium 4. Carbon. Fluorine 6. Neon. Magnesium 8. Chlorine. Arsenic 10. Krypton. Barium 12. Tin To draw the Bohr model diagram for any atom, first find the number of protons, electrons, and neutrons of the atom, then, draw the nucleus and write proton and neutrons at the center of it, after that, draw the first electron shell, second electron shell, third electron shell, etc. depending on the availability of electrons. Bohr Diagram write the nucleus with the atomic symbol, number of protons and neutrons; draw a circle around the whole thing; either draw electron shells with the amount of electrons or write the number of electrons in parenthesis used in place of electron shells
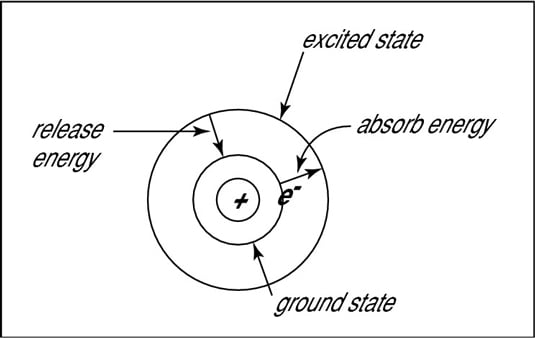
draw energy level diagram of hydrogen atom showing different series of lines in its spectrum; ... The next attempt to suggest a model for atom was made by Neils Bohr- a student of Rutherford. This model used the concept of quantisation of energy of electrons in the atom. Since this fact was suggested by line spectrum of hydrogen atom it is worthwhile to understand the meaning of a spectrum ...
How to draw the Bohr-Rutherford Diagram for Germanium. The order of filling makes Bohr-Rutherford Diagrams for Elements beyond #20 (Calcium) tough. 2 in th...
Draw a Bohr Model of Beryllium Draw a Bohr Model of Chlorine Activity. E- 11 electrons. N- 12 neutrons.Name Period Date Bohr Model Diagrams. Use the information provided for each element to draw Bohr Model diagrams. Rather than drawing individual protons and neutrons, you may simply label how many of each there are in the nucleus (e.g ...
How to draw a Bohr model of an atom... Symbols to take note of: p⁺ :symbol for protons n⁰ :symbol for neutrons e⁻ :symbol for electrons.6 pages
The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the third layer. This is because the atomic number of Potassium (K) is 19, therefore has 19 protons and 19 electrons. == The phosphorus bohr model has 3 ...
so in the last video I showed you how to get this equation using a lot of physics and so it's actually not necessary to watch the previous video you can just start with this video if you want and E one we said was the energy associated with an electron and the lowest energy level of hydrogen and we're using the Bohr model and we calculated the value for that energy to be equal to negative two ...
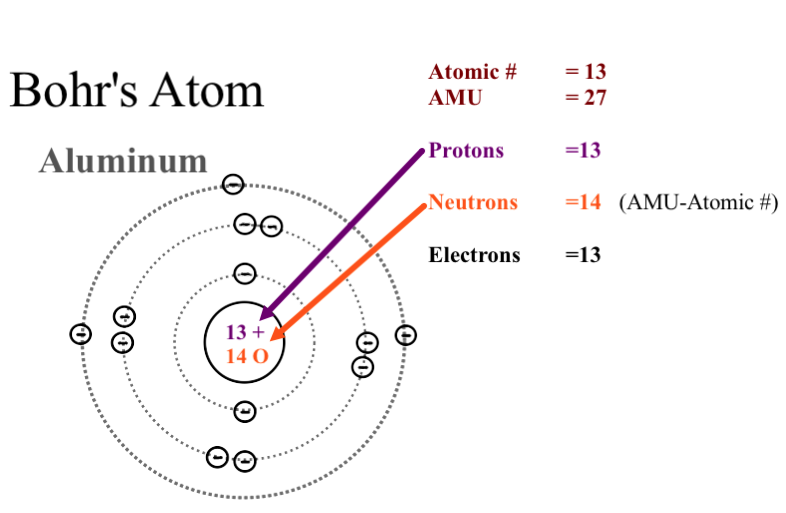
To draw a Bohr model of an atom, first find the number of protons, neutrons and electrons in the atom from its atomic weight and atomic number. After that, place the neutrons and the protons in the nucleus, and draw the electrons in their designated shells. From the periodic table, find the element, and identify its atomic number and atomic ...
Bohr Diagrams 1) Draw a nucleus with the element symbol inside. 2) Carbon is in the 2ndC period, so it has two energy levels, or shells. 3) Draw the shells around the nucleus. 5. Bohr Diagrams 1) Add the electrons. 2) Carbon has 6 electrons.C 3) The first shell can only hold 2 electrons. 6.
15 Aug 2020 — The Bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific ...
Draw A Sketch Of Bohr S Model Of An Atom With Three Shells From Science Structure Of The Atom Class 9 Uttarakhand Board
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule making a transition from a high energy state to a lower energy state. The photon energy of the emitted photon is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each ...
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Drawing Bohr Models 3. Draw the first shell 4. Add the electrons, the first shell can only hold 2 P:9 N:10. Drawing Bohr Models 5. Draw the next shell if you have more electrons to add P:9 N:10. Drawing Bohr Models 6. Add your electrons, the 2nd shell can hold up to 8 Be sure to add them one at a time
14 Bohr Diagrams Add the electrons. Carbon has 6 electrons. The first shell can only hold 2 electrons. To correctly draw the dots… Draw an X over the diagram ...
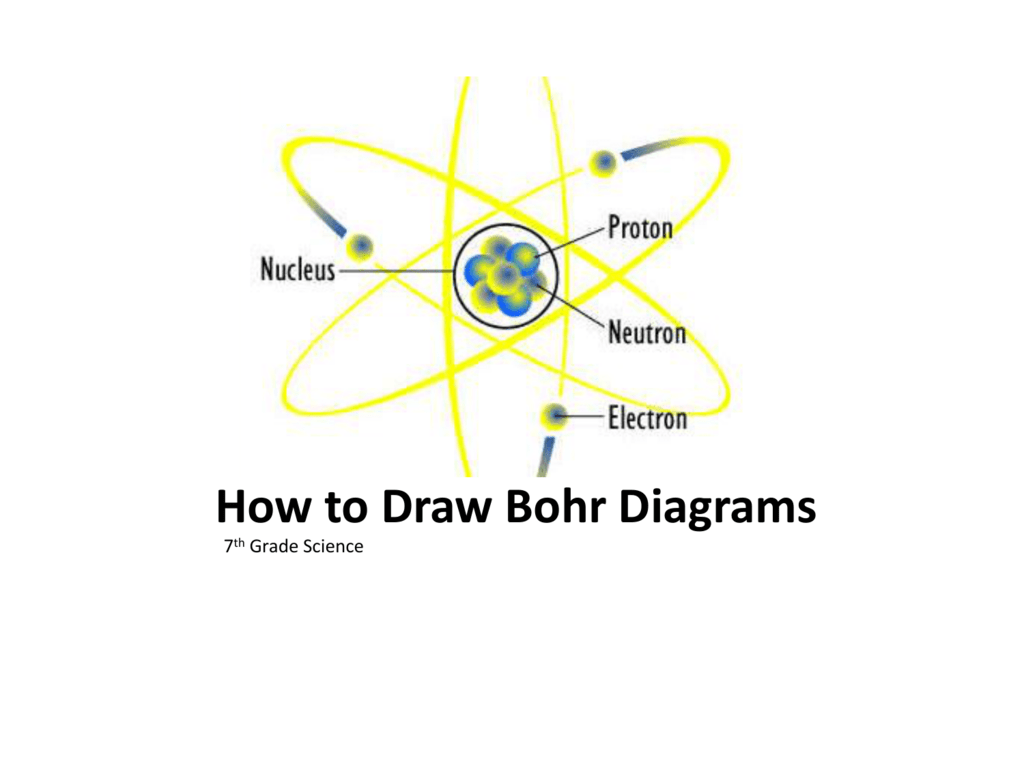
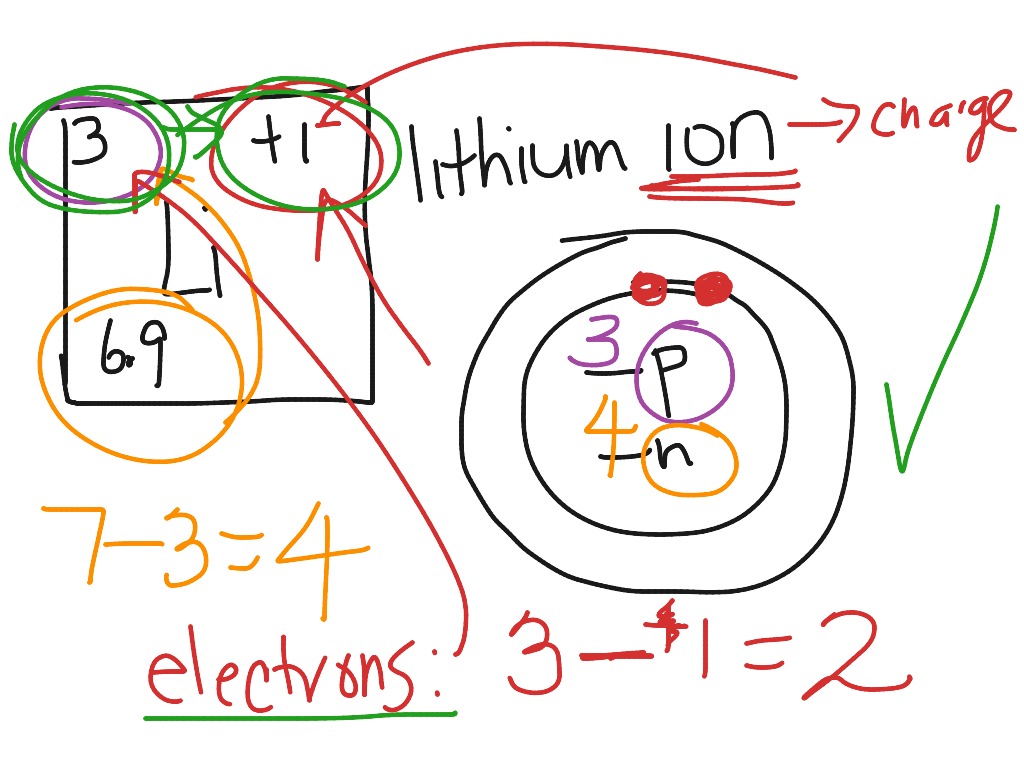
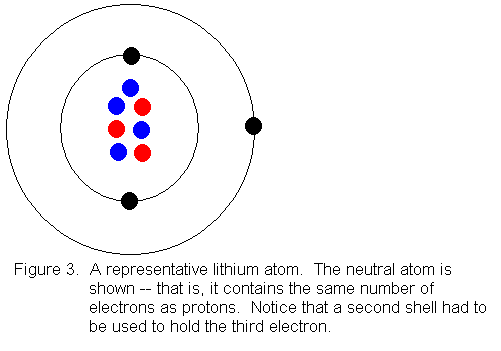
In atomic physics, the Rutherford-Bohr model or Bohr model or Bohr diagram, presented by Niels Bohr and Ernest Rutherford in , is a system consisting of a small, dense nucleus surrounded by revolving electrons —similar to the structure of.draw a Bohr-Rutherford diagram for lithium. draw a Bohr-Rutherford diagram for beryllium. draw a Bohr ...
29.09.2021 · An electron dot diagram is a representation of an atom’s valence electrons that employs dots to surround the element’s symbol. The number of dots corresponds to the atom’s valence electrons. With no more than two dots on each side, these dots are positioned to the right and left, above and below the symbol. How to Draw Electron Dot Diagram? The steps that must be followed while drawing a ...
how to draw bohr diagrams michelle bartels bohr diagrams 1 draw a nucleus with the number of protons and neutrons inside 2 carbon is in the 2nd period so it has two energy levels or shells. blank bohr model worksheet blank fill in for first 20 elements.
Bohr Diagrams 1) Draw a nucleus with the number of protons and neutrons inside. 2) Carbon is in the 2nd period, so it has two energy levels, or shells. 3) Draw the shells around the nucleus. 6p 6n. Bohr Diagrams 1) Add the electrons. 2) Carbon has 6 electrons. 3) The first shell can only

How To Draw Bohr Rutherford Diagrams Powerpoint Elements 1 20 Chemistry Mrs Brosseau S Binder On Teac Teaching Chemistry Chemistry Teaching Homeschool
Bohr Diagrams 1) Carbon is in the 2nd period, so it has two energy levels, or shells. 2) Draw the shells around the nucleus. C P+ = 6 N0 = 6. 7. Bohr Diagrams 1) Add the electrons. C P+ = 6 N0 = 6 2 e- 2) Carbon has 6 electrons. 3) The first shell can only hold 2 electrons.
How to draw bohr diagrams slideshare 1. Atomic structure atoms have a nucleus that contains protons and neutrons electrons are. Lets take a look at how to draw bohr diagrams. Find out which period row your element is in. Draw an arc to represent the first energy level. Draw a circle to represent the nucleus of the atom.
How Do You Draw A Bohr Rutherford Diagram; Write the number of neutrons and the number of protons in the nucleus. Draw the first energy level. Draw the electrons in the energy levels according to the rules below. Keep track of how many electrons are put in each level and the number of electrons left to use.. Likewise, how do Bohr diagrams work?
Review what a Lewis dot diagram is and discover how to draw a Lewis dot structural formula for compounds. Learn how to represent single, double and triple bonds with lines instead of dots.
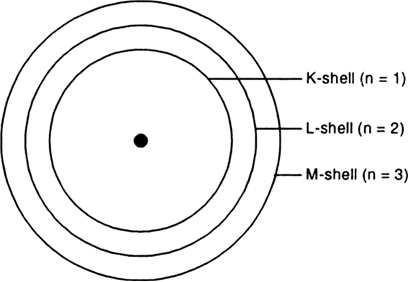
Using the Main Group Elements of the Periodic Table to Draw Bohr-Rutherford Diagrams He. Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus ...
Visit http://www.makemegenius.com for more free science videos for kids.What is an Atom? A good video explaining atomic structure & molecules formation. An a...
Bohr model of the first 20 elements. Due to the technical limitations of my software, I will list the number of protons and neutrons of elements. In this model of the atom, the electrons travel around the nucleus in well-defined Steps for Drawing Bohr Diagrams (for use only with the first 20 elements). 1.
The Bohr model works well for very simple atoms such as hydrogen (which has 1 electron) but not for more complex atoms. bohr rutherford diagram drawing task cards exit cards - 28 images - grade stuff on middle school science, ppt differentiated technology day best images about chemistry on activities, watches on, how to draw a bohr rutherford ...
These are what make up the Periodic Table of Elements: This is how a Bohr-Rutherford Diagram look like and this is Nitrogen: 1) There are 10 electrons in Neon. This is how you Calculate Protons, Neutrons, and Electrons in an Atom: This is how you draw the Bohr-Rutherford Diagram: 1) There are 19 electrons in Potassium.
Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. This is because protons and neutrons ...
















0 Response to "35 how to draw a bohr diagram"
Post a Comment