35 nf molecular orbital diagram
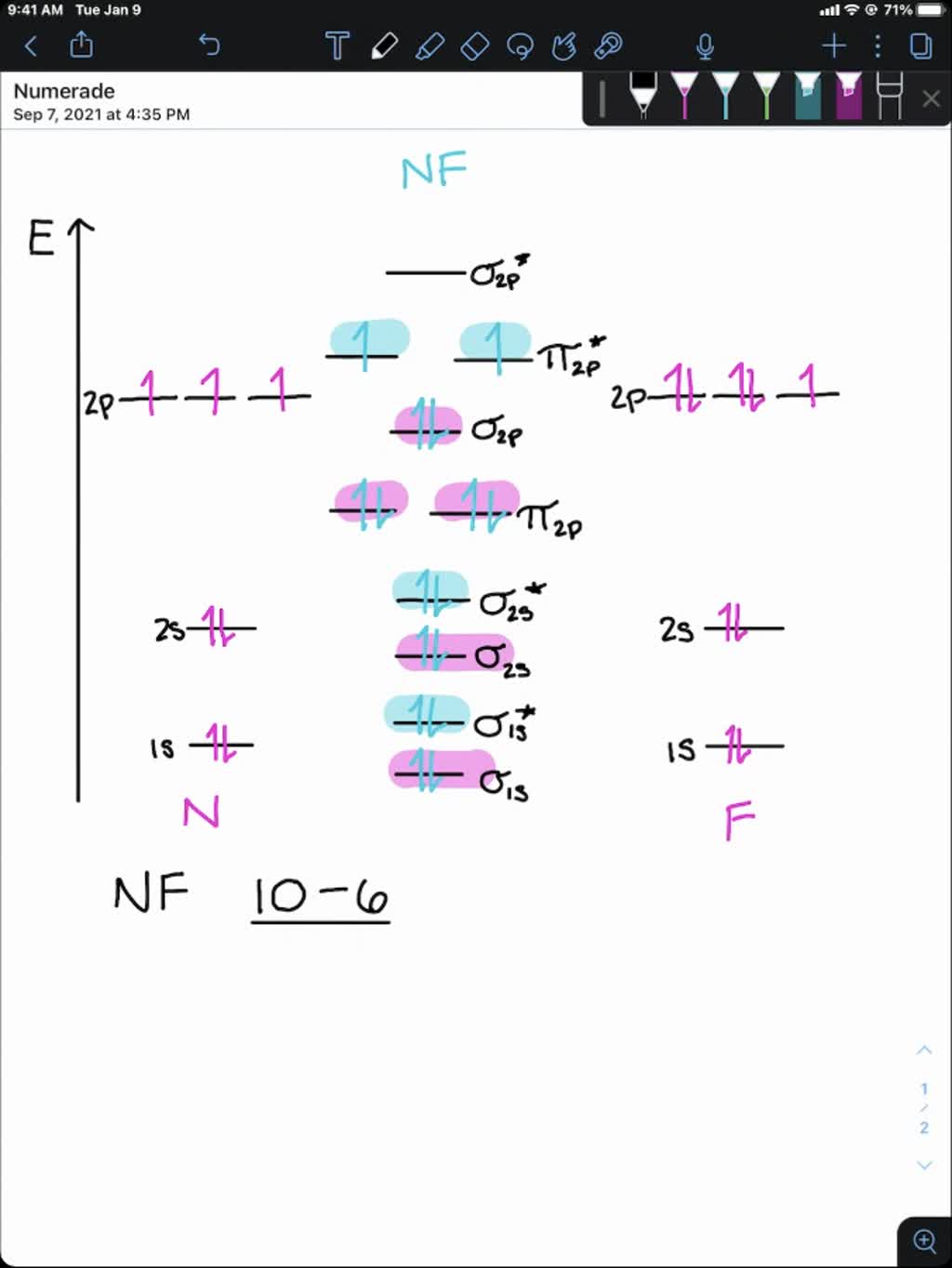
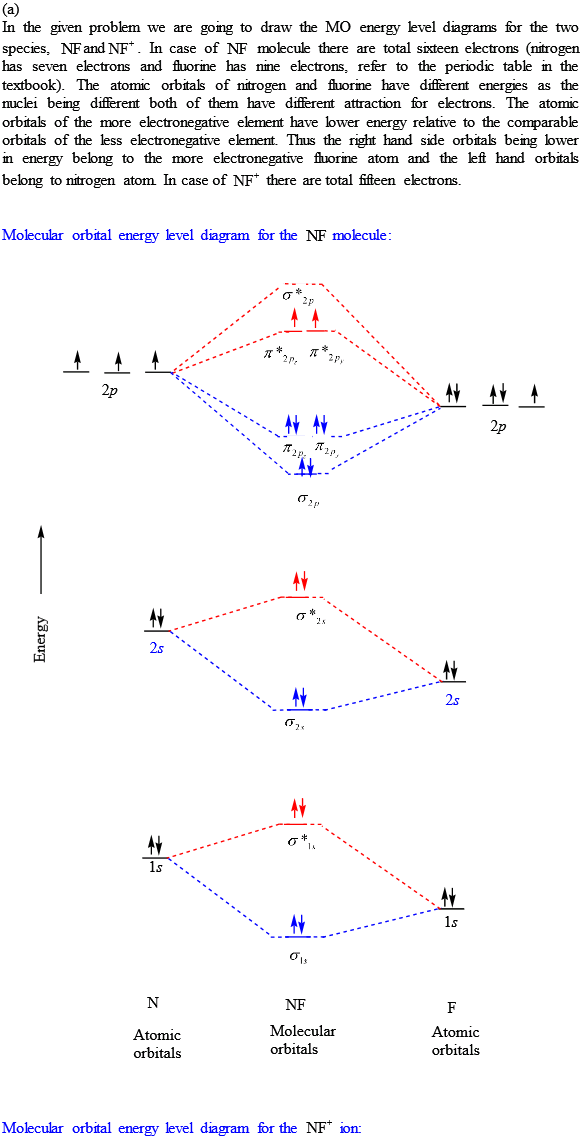
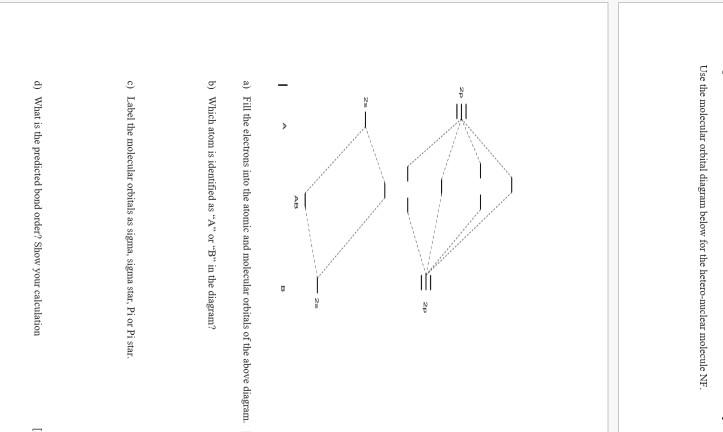
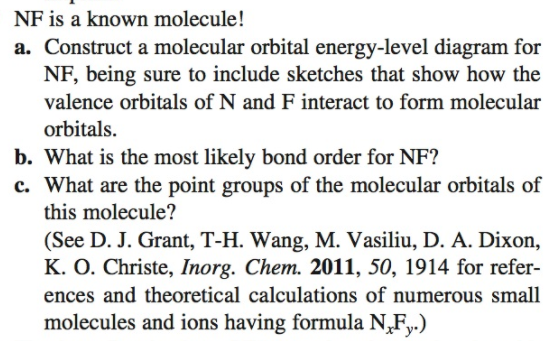
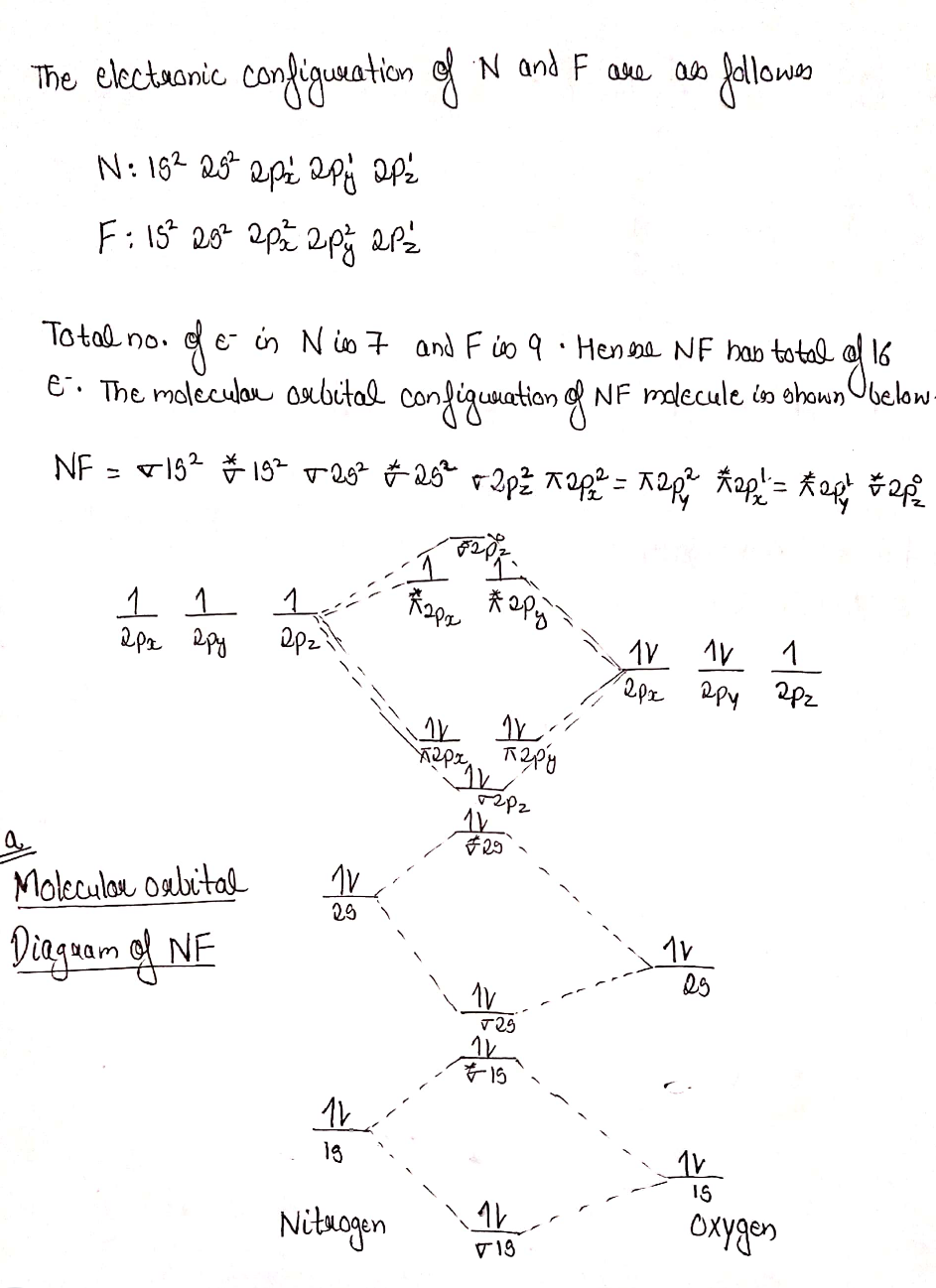
NF is a known molecule. Construct a molecular orbital energy-level diagram for NF, being sure to include sketches that show how the valence electrons orbital interact to form molecular orbitals. What is the most likely bond order of NF. Orbital energies ( N: 2s = -25.56 eV, 2p = -13.18 eV, F 2s = -40.17 eV, 2p = -18.65 eV). Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
molecular orbitals. 2.The number of molecular orbitals formed is the same as that of the number of atomic orbitals combined. 3.The additive overlap results in the bonding molecular orbital while the subtractive overlap results in the antibonding overlap. 4.The energy of bonding molecular orbitals is lower than their nonbonding counterparts ...
Nf molecular orbital diagram
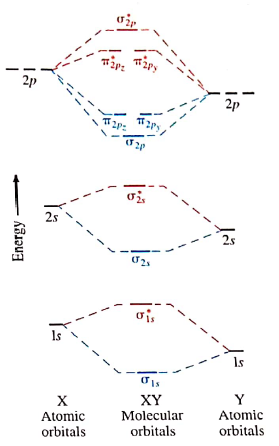
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
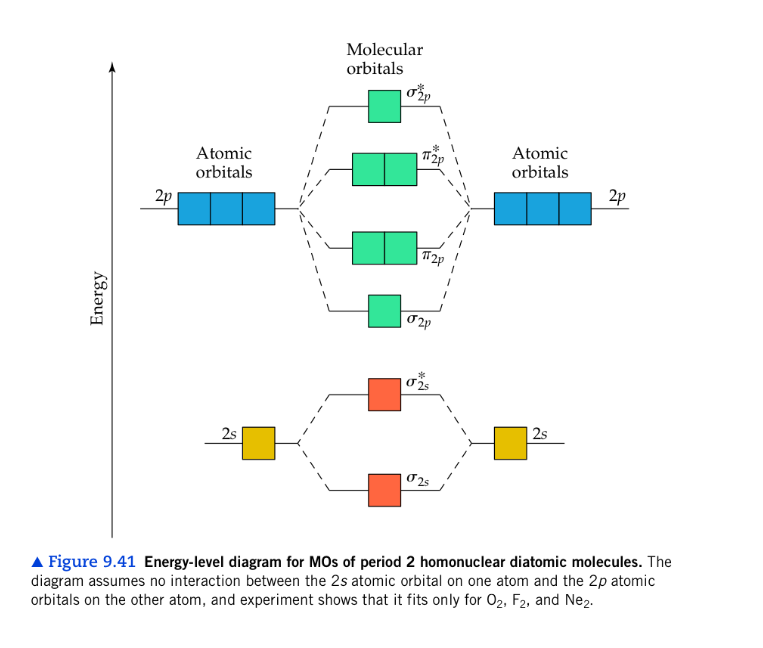
Nf molecular orbital diagram. #"O"_2# is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that #"CO"# is not (as it has zero unpaired electrons), but #"NO"# is (it has one unpaired electron). Well, the MO diagram for #"O"_2# is: The bond order is already calculated in the diagram. ©2021 Prof Adam J Bridgeman | close window : ©2021 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window Molecular Orbital Diagram for NF. Post by Christine Van 2E » Thu Nov 05, 2015 6:35 am . Hi! I have a quick question about the molecular orbital diagram for NF. Lavelle said that whenever we have a heteronuclear molecule, if one or both atom(s) has Z<8, then we would use the diagram where the pi px and pi py are lower than the Sigma pz. However ... Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
NF is a known molecule! a. Construct a molecular orbital energy-level diagram for $\mathrm{NF},$ being sure to include sketches that show how the valence orbitals of $\mathrm{N}$ and $\mathrm{F}$ interact to form molecular orbitals. b. What is the most likely bond order for NF? c. What are the point groups of the molecular orbitals of this ... In Table 5 we give the dominant electron configuration for each state in terms of natural orbitals (15), i.e., the orbitals which diagonalize the first order reduced density matrix. These orbitals are in this case molecular orbitals, which can be given in terms of the raw atomic Slater type basis, as is done in Table 6 for the X 3-'- state of NF. 4. Draw the valence molecular orbital diagram for NF. State the bond order, the molecular orbital configuration and determine whether each of the following molecules/ions is paramagnetic or diamagnetic. (6 points) a) Molecular Orbital Diagram b) Bond Order _____ c) Molecular orbital configuration _____ d) Consider NF, NF +, and NF-. Which of ... Structure and Bonding Solutions: #4. 4.* (1997 1 7) Consider the diatomic molecule NF. Draw its molecular orbital energy diagram. A. Using the LCAO-MO scheme, indicate the ground-state MO description for NF, i.e. complete the following: 1(s) 2. . .The LCAO-MO description is: 1s 2 2s 2 3s 2 4s 2 5s 2 1p 4 2p 2. B.
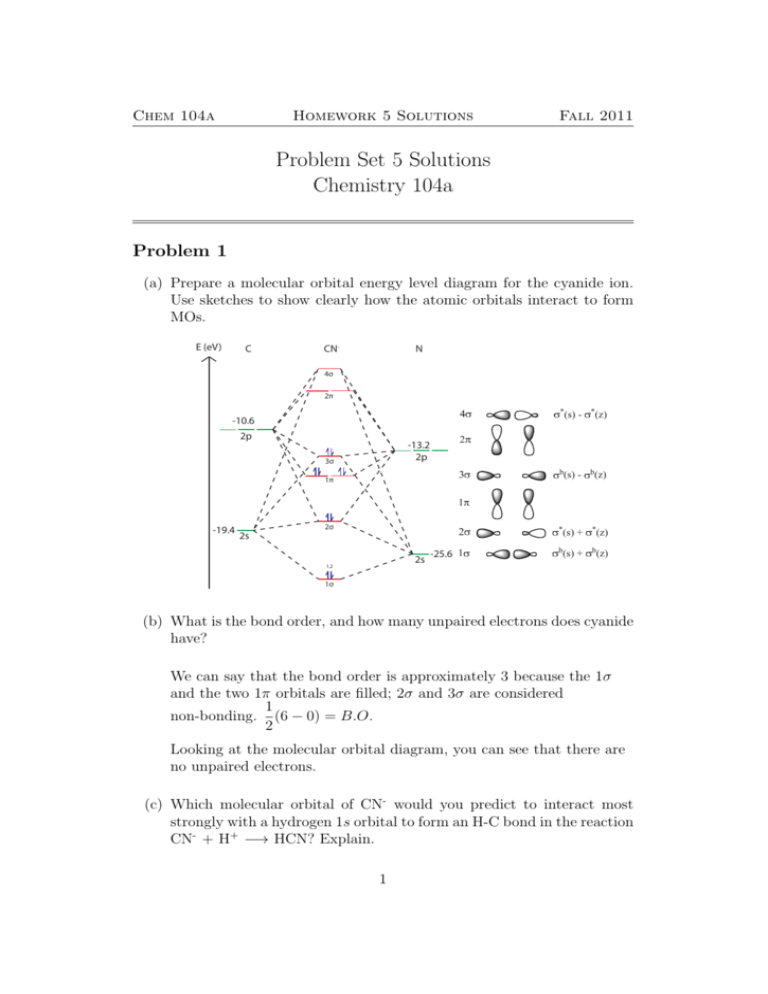
The molecular orbital diagram for ClO - is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated. Sun, 31 Oct 2021 #LOCANEX, Nf Molecular orbital diagram - Wikipedia /wiki/Molecular_orbital_diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as ... Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. ANSWERS TO MOLECULAR ORBITALS PROBLEM SET 1. (a) N2 +(13 e-): σ2 1sσ*21sσ22sσ*22sπ22pπ22pσ12p N2 2+(12 e-): σ2 1sσ*21sσ22sσ*22sπ22pπ22p N2 (14 e-): σ2 1sσ*21sσ22sσ*22sπ22pπ22pσ22p N2-(15 e-): σ21sσ*21sσ22sσ*22sπ22pπ22pσ22pπ*12p N2 2-(16 e-): σ21sσ*21sσ22sσ*22sπ22pπ22pσ22pπ*12pπ*12p (b) Bond orders are: N2 + = 2.5 ; N 2 2+ = 2.0 ; N
NF3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. Nitrogen trifluoride or NF3 is a nitrogen halide compound that is slightly water-soluble. Its noticeable characteristics include being colorless and carrying a musty or moldy odor. NF3 has a molar mass of around 71.002 g/mol and a density of 3.003 kg/m3.
You will recall that molecular orbitals form in bonding and antibonding combinations; hybrid orbitals have equivalent energies. For example, the wavefunctions for the two sp hybrids are of the form Y = (1/2) 1/2 (Y 2s + Y 2p) or Y = (1/2) 1/2 (Y 2s - Y 2p). The two orbitals are equivalent. Note the shape and signs of the lobes for the ...
Click Images to Large View Trip Epiphanies Visualising String Theory Whilst Off Your. Feminist. 02 Molecular Orbital Diagram. MO Diagram for F2. Nodes Molecular Orbitals. BH3 MO Diagram. Adult Learning. Human Behavior. Valence Bond Theory Examples.
Draw a molecular orbital diagram for NF and calculate the bond order. Also, comment on its magnetic property. close. Start your trial now! First week only $4.99! arrow_forward. Question. Draw a molecular orbital diagram for NF and calculate the bond order. Also, comment on its magnetic property. check_circle Expert Answer. star. star. star.
In this screencast, Andrew Burrows walks you through how to construct the MO energy level diagram of HF. http://ukcatalogue.oup.com/product/9780199691852.do#...

High Spin Iron Vi Low Spin Ruthenium Vi And Magnetically Bistable Osmium Vi In Molecular Group 8 Nitrido Trifluorides Nmf3 Stuker Chemistry A European Journal Wiley Online Library
NF is a known molecule! Construct a molecular orbital diagram for NF, an include sketches showing how the valence orbitals of N and F interact to form MOs.
Therefore, NF is predicted to be paramagnetic with a bond order of 2. The populations of the bonding (8 electrons) and antibonding (4 electrons) molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p *
The valence orbitals (such as and orbitals) of N and F interact to form a molecular orbital of. The energy levels of oxygen are low lying since oxygen is more electronegative than nitrogen. The molecular orbital energy-level diagram for is shown as follows: Chapter 5, Problem 9P is solved.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. In this answer of Martin's, you can find a molecular orbital diagram of $\ce{CO}$.

Theories Of Bonding And Structure Chapter 10 Chemistry The Molecular Nature Of Matter 6 Th Edition By Jesperson Brady Hyslop Ppt Download
From this I built A1 bonding and antibonding orbitals, A'2 bonding and antibonding orbitals, 1 A'2 and 2 E''2 non bonding orbitals. I'm now stuck because I have 4 E' on the F side and only 2 E' on the B side. I found the following diagram for BF 3 online but it doesn't generate the E' anti bonding and also doesn't generate enough molecular ...

Given That O2 Is Paramagnetic And Has A Bond Order Of 2 And Its Highest Occupied Molecular Orbital Is Antibonding What Would Be The Expected Bond Orders For O2 2 And O2 2
For each of the two species NF and NF +, (a) draw MO energy level diagrams; (b) write out electron configurations; (c) determine bond orders and predict relative stabilities; and (d) predict diamagnetism or paramagnetism. Step-by-step solution. Step 1 of 5. Chapter 9, Problem 34E is solved. View this answer.
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Solved 12 7 Sketch The Molecular Orbital Diagrams For The Following Nz 0z Cz Fz Cn No Which Of These Species Would You Expect To Become More Stable If A An Electron Is Added And
Consider The Following Molecules No No And No Using The Molecular Orbital Theory How Do You Evaluate Them In Terms Of Bond Energy And Stability Quora

Draw The Molecular Orbital Energy Diagram For Oxygen Molecule O2 And Show That I It Has A Double Bond Ii It Has Paramagnetic Character From Chemistry Chemical Bonding And Molecular Structure Class 11 Cbse

Second Order Jahn Teller Interactions At Unusually High Molecular Orbital Energy Separations Dalton Transactions Rsc Publishing Doi 10 1039 D0dt00137f














0 Response to "35 nf molecular orbital diagram"
Post a Comment