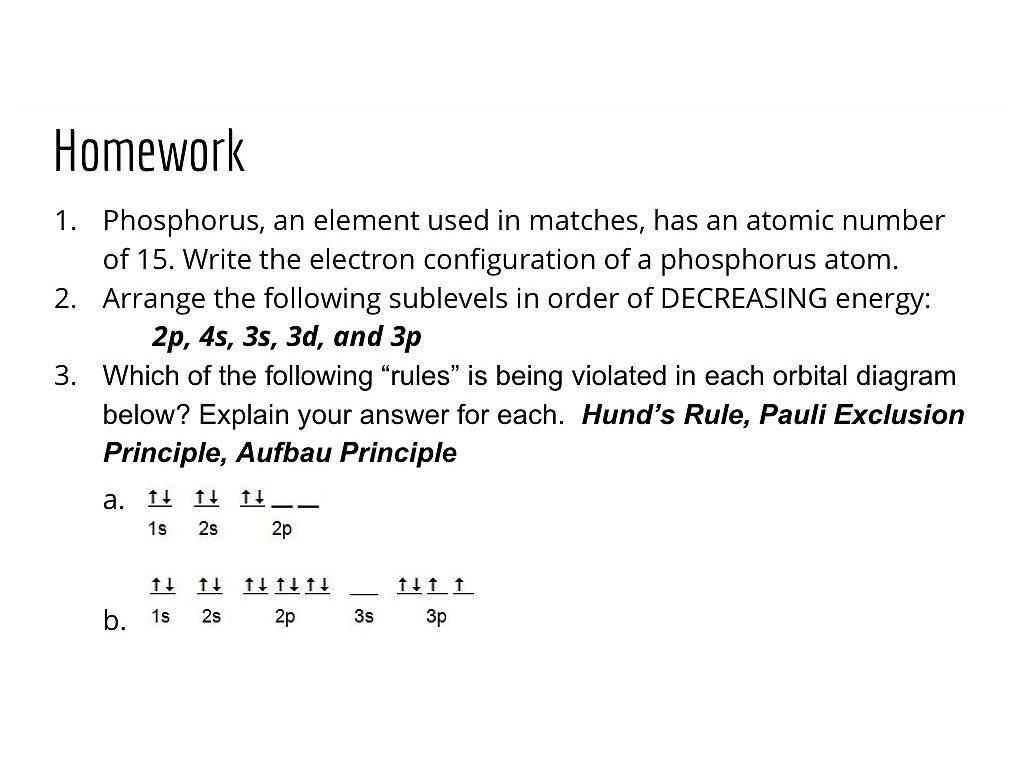
35 orbital diagram of phosphorus
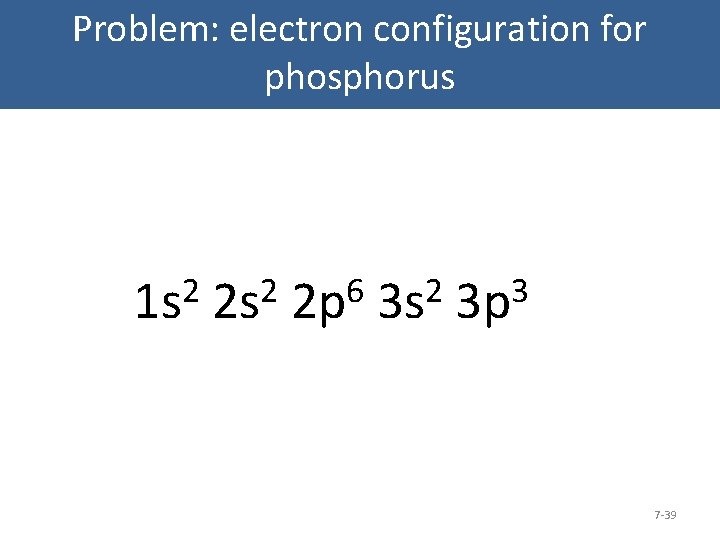
There are alkali, argon, electron, nitrogen, phosphorus, silicon, and sulfur orbital diagrams that you can save for free. Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. Orbital diagram. Phosphorus electron configuration ← Electronic configurations of elements . P (Phosphorus) is an element with position number 15 in the periodic table . Located in the III period. Melting point: 44 ℃. Density: 1.82 g/cm 3. Electronic configuration of the Phosphorus atom: 1s 2 2s 2 2p 6 3s 2 3p 3 Reduced electronic configuration P: [Ne] 3s 2 3p 3. Below is the electronic ...
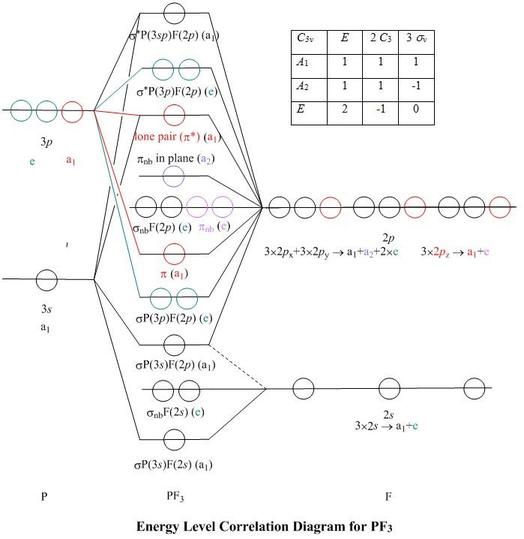
Irrespective of it, the phosphorus trifluoride (PF3) shows pi bonding characteristics due to sp3 hybridization and back-bonding. The detail of how hybridization is taking place can be studied through the molecular orbital diagram of phosphorus trifluoride (PF3) molecule.

Orbital diagram of phosphorus
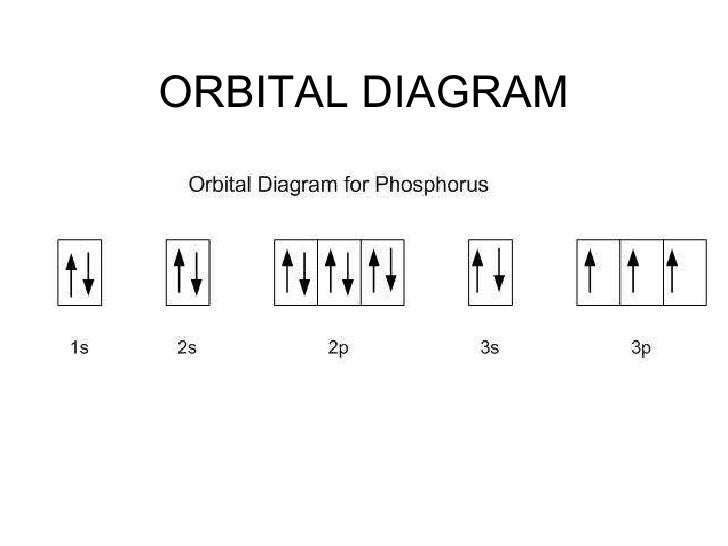
There are three unpaired electrons. As you can see in the electron configuration, the 3p sublevel has room for three more electrons. The orbit filling diagram and the electron dot diagram show the empty spaces for three more electrons and how there are three electrons that aren't paired. Orbital diagram phosphorus answers an orbital diagram is similar to electron configuration except that instead of indicating the atoms by total numbers each orbital is shown with up and down arrows to represe nt the electrons in each orbital refer to the link to see an illustration of an orbital diagram for aluminum. Box spin diagram of outer electron orbitals for the electron configuration of ... Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus (P) 16: Orbital diagram of Sulfur ...
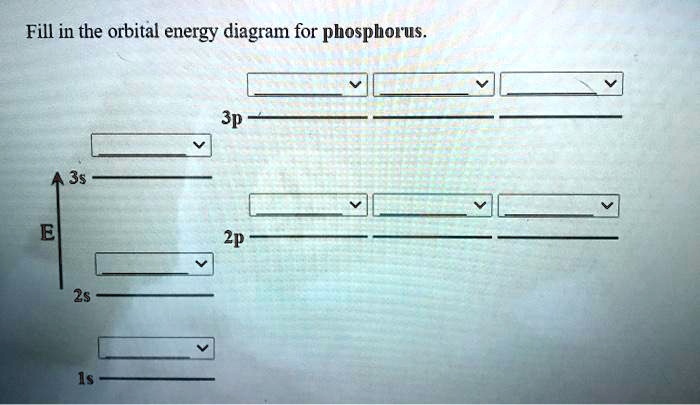
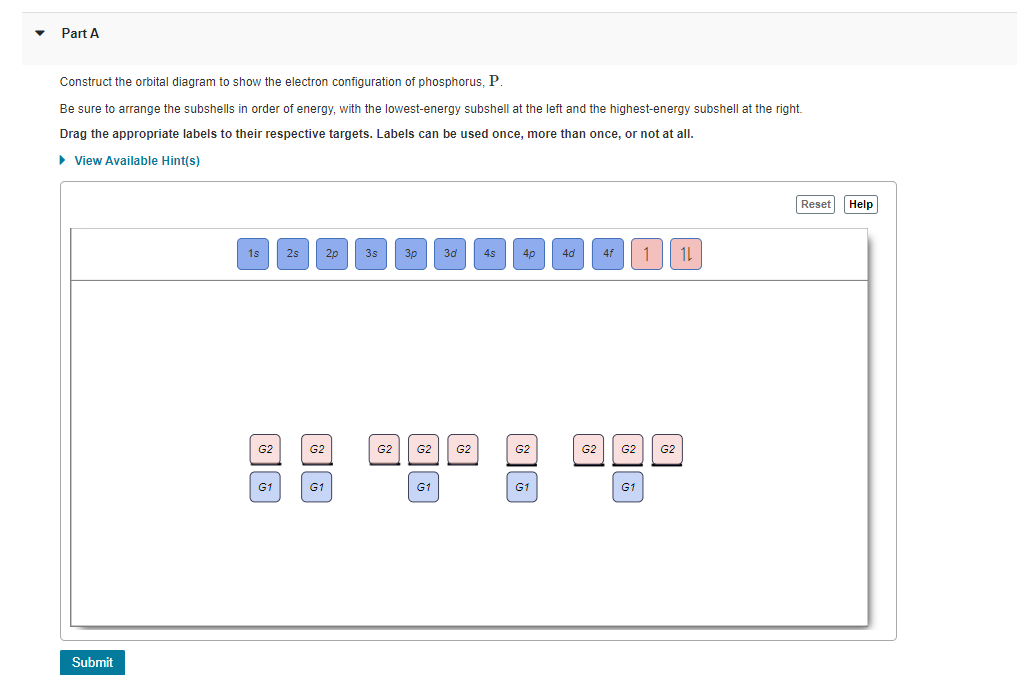
Orbital diagram of phosphorus. Complete the atomic orbital diagram for the ground-state electronic configuration of phosphorus. 3p C00 Answer Bank Answer Bank Energy 2,0 ; Question: Complete the atomic orbital diagram for the ground-state electronic configuration of phosphorus. 3p C00 Answer Bank Answer Bank Energy 2,0 What is the orbital diagram for phosphorus? In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. You are watching: Use the orbital-filling diagram to show the electron configuration of phosphorus, p. Electron Configuration. Electron configurations room the an overview of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons same to its variety of protons. 21 Jan 2021 — Phosphorous is a chemical element that has an atomic number of 15. Its electron configuration with regards to electrons present in each shell is ...
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three guidelines are helpful in forming orbital diagrams. In response to the Auf Bau Precept, every electron occupies the bottom vitality orbital. You leap up a bit bit in vitality and we get the 2s orbital that make it the 2p sublevel. Build the orbital diagram for the ion most likely formed by phosphorus? 1s22s22p63s23p3 is for Phosphorus and the most likely ion is to be a 3- because it wants to have a full outer shell ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... Question : What is the orbital diagram for phosphorus. Related Answer. More Related Question & Answers. In the molecular orbital diagram for O_2^(+) ion the ...
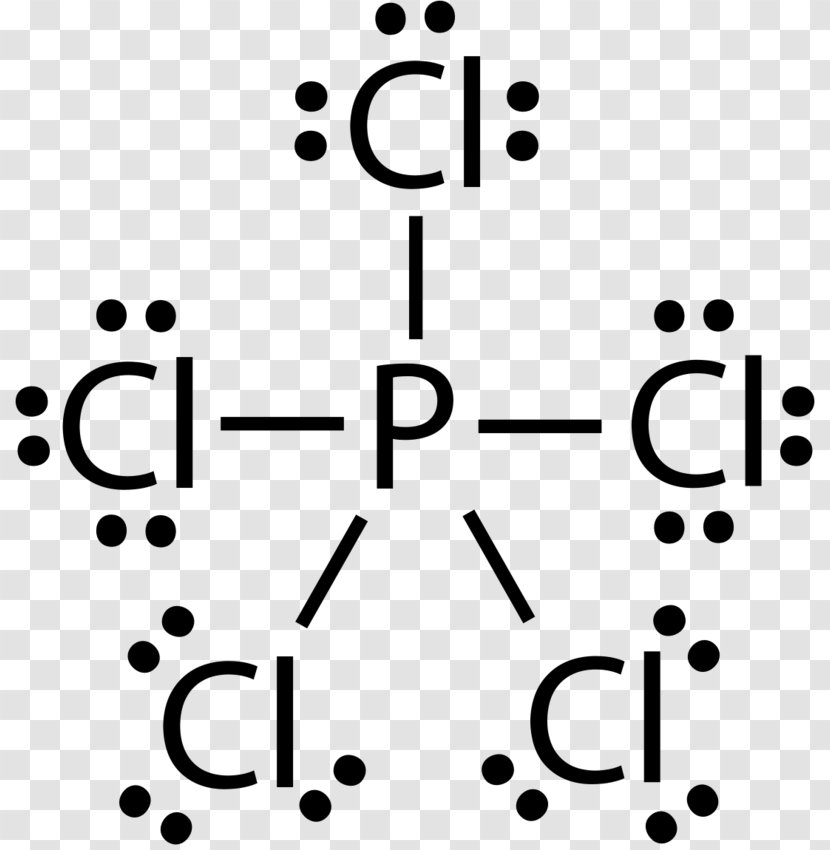
Oxidation States, -3. Electrons Per Shell, 2 8 5. Electron Configuration, [Ne] 3s2 3p3. 1s2 2s2 2p6 3s2 3p3. Orbital Diagram. The lone pair orbital is mainly the s orbital. Phosphorus forms three bond pairs and one lone pair. However, the whole concept can be better explained if you understand Drago's Rule. This rule states that hybridization will not take place if; Central atom belongs to third or higher period. This could have been a problem, but it can hold the 5 Chlorine atoms, due to its empty 3d orbital. Step 5: Visualizing the diagram, we come up with a Phosphorus in the center, housed by 5 Chlorine atoms. Molecular Geometry of PCl5. Molecular geometry is an extension of the 2-dimensional diagram as in the below image. What is the correct orbital diagram for phosphorus? The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s22s22p63s23p3.

Select The Correct Statement Below A Phosphorous Contains 10 Core Electrons And 5 Valence Brainly Com
A molecular orbital diagram or MO diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the Linear combination of atomic orbitals molecular orbital method (LCAO method) in particular.schematron.org: Phosphorus: Orbital and Bonding InfoWhat is the orbital ...
Orbital Diagrams: Orbital diagrams show the distribution of electrons in the electron shells and subshells of an atom. A few principles and rules must be followed in this process.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
What is the atomic structure of phosphorus? Diagram of the nuclear composition and electron configuration of an atom of phosphorus-31 (atomic number: 15), the most common isotope of this element. The nucleus consists of 15 protons (red) and 16 neutrons (blue). 15 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).
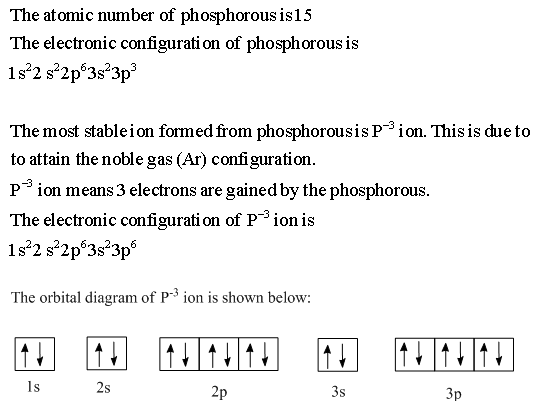
It has 5 valence electrons in its valence shell. The orbitals involved in phosphorus atoms are 1s, 2s, 2p, 3s and 3p. The outermost orbitals, 3s and 3p, have 5 ...1 answer · Top answer: The atomic number of phosphorus is 15. It has 5 valence electrons in its valence shell. The orbitals involved in phosphorus atoms are 1s, 2s, 2p, 3s and ...
Use the orbital-filling diagram to show the electron configuration of helium, He. ... phosphorus silver molybdenum. phosphorus germanium silver molybdenum strontium. Arrange the following elements in order of decreasing metallic character (high to low): Cl Cs Sr Rh Se Mo As Cd. Cs Sr Mo Rh Cd As Se Cl.
PLEASE HELP! Write the full electron configuration for phosphorus, atomic symbol P, then draw an orbital box diagram that accounts for all of the electrons in phosphorus. You don't need to include the orbital box diagram as part of your answer. Based on your drawing, explain why phosphorus is either paramagnetic or diamagnetic.
The aufbau diagram shows the. The atomic number of phosphorus is Write the electron configuration of a phosphorus atom. 1s22s22p63s23p3. You can obtain correct electron configurations for the elements up to. In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital.
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now ...
Orbital Diagram Of Phosphorus. what is the orbital diagram for phosphorus the orbital diagram for phosphorus consists of five electrons in the third shell eight in the second and two in the first shell closest to the nucleus electron configuration for phosphorus p the next six electrons will go in the 2p orbital the p orbital can hold up to six electrons we ll put six in the 2p orbital and ...
Transcribed image text: Question 17.a of 25 Submit Examine the orbital diagram for the ground state electron configuration of phosphorus. Choose the correct orbital diagram for the ground state electron configuration of phosphorus. 1) [Nej 11 3s Зр A) II) [Ne) 11 B) II 3s 3p [Ne] C) III 11 3s 3p D) IV IV) [Ne] 11 + 3s 3p
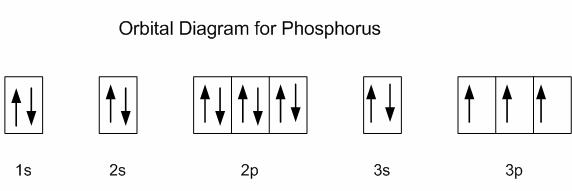
Draw orbital diagrams for the following elements: 1. phosphorus. ↑↓. ↑↓. ↑↓ ↑↓ ↑↓. ↑↓. ↑ ↑ ↑. 1s. First, he Aufbau principle requires that lower energy orbitals are filled with Since phosphorus in a third period element, the first (K) and second (L) shells are . Phosphorus, P, is located in period 3, group 15 of ...
Clearly, it is a phosphorus sp mixture orbital, with a visible p-type inner lobe, and contains, according to the present calculation, 37% P(3s), 32% P(3p z) and 9% from each fluorine 2p z The highest energy valence-shell MO, labelled as σ * P(3 sp )F(2 p ), has more phosphorus p -character, and contains 19% P(3 s ) and 43% P(3 p z ).
Part B Build The Orbital Diagram For The Ion Most Likely Formed By Phosphorus Use The Buttons At The Top Of The Tool Home Work Help Learn Cbse Forum
30 May 2020 — The electron configuration for phosphorus is 1s2 2s2 2p 6 3s 2 3p 3 and the orbital diagram is drawn below. orbial diag phosphorus.png. Back to ...

I Think The Answer Is B As Phosphorus Has Vacant D Orbitals And It Can Use It To Accept Electron From Carbon But Can It Not Be Chlorine Since It Also Has
Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus (P) 16: Orbital diagram of Sulfur ...
Orbital diagram phosphorus answers an orbital diagram is similar to electron configuration except that instead of indicating the atoms by total numbers each orbital is shown with up and down arrows to represe nt the electrons in each orbital refer to the link to see an illustration of an orbital diagram for aluminum. Box spin diagram of outer electron orbitals for the electron configuration of ...
There are three unpaired electrons. As you can see in the electron configuration, the 3p sublevel has room for three more electrons. The orbit filling diagram and the electron dot diagram show the empty spaces for three more electrons and how there are three electrons that aren't paired.
Use The Orbital Filling Diagram To Show The Electron Configuration Of Phosphorus P Wiring Site Resource

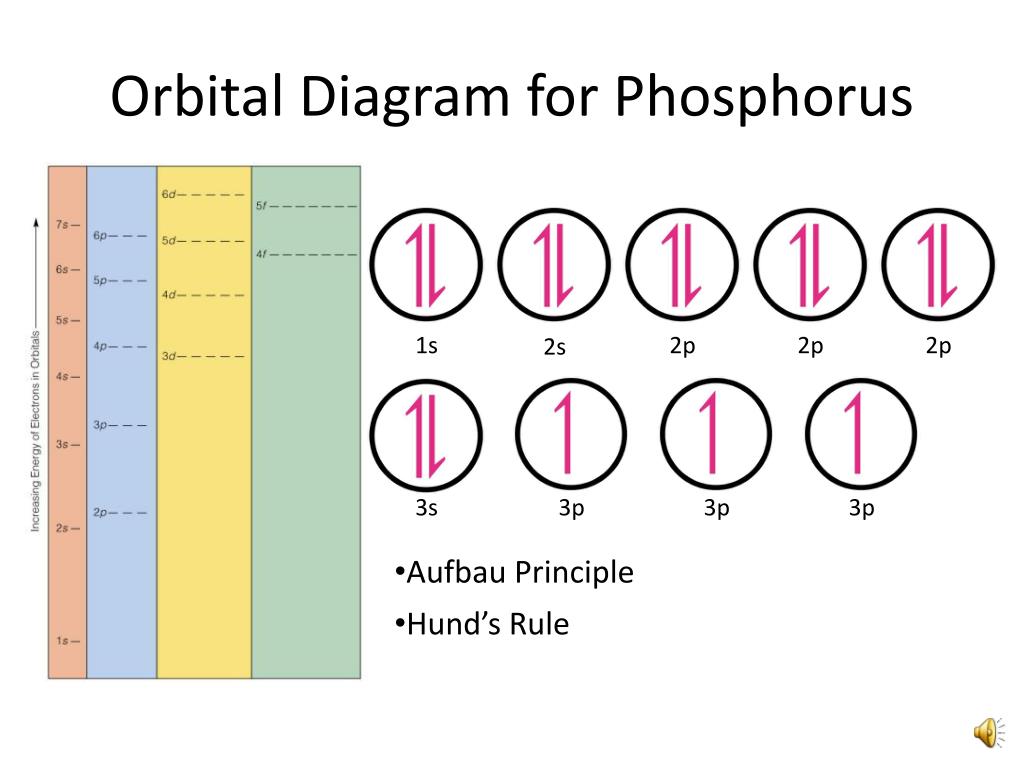
Orbital Diagrams Hund S Rule Since Electrons Repel They Want To Stay As Far Away From Each Other As Possible So They Occupy Different Orbitals Until Ppt Download

















0 Response to "35 orbital diagram of phosphorus"
Post a Comment