35 phase diagram of nitrogen
In this work, we have calculated those thermodynamic properties using density-functional theory (DFT) for Fe4N, Fe16N2 and ferrite with nitrogen ... The phases and phase transformations in the argon—nitrogen system have been determined by x-ray diffraction with results very different from previous published phase diagrams. The solid phase in ...
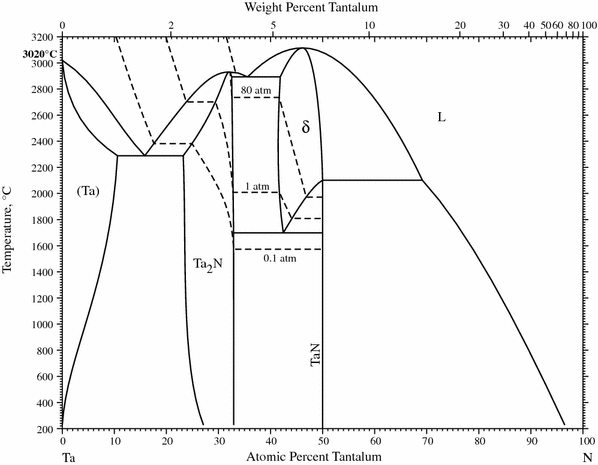
11 Sept 2020 ... We investigate the pressure–temperature phase diagram of the tantalum–nitrogen system through a combination of density functional theory ...

Phase diagram of nitrogen
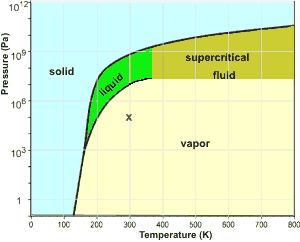
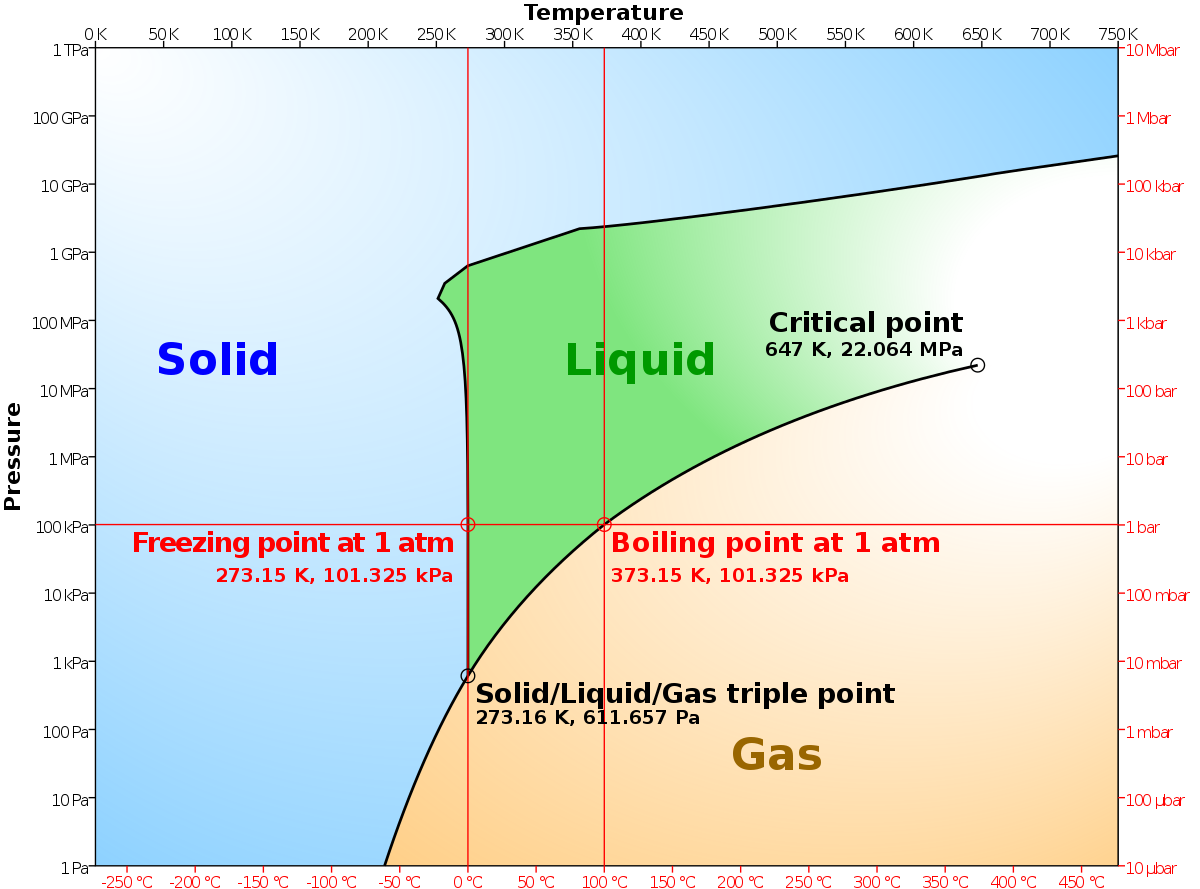
C. Boron carbon nitrogen ternary Little experimental data exist on the B-C-N ternary. Previous phase diagrams are either presented at very high temperature or lack detailed information, especially at the high boron corner [33]. No stable compounds have been reported in the B-C-N ternary system. However, variants of diamond/c-BN such as BC 2N and BC Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ... From the densities of liquid nitrogen and nitrogen gas at standard pressure the volume ratio is about 1:700. For an ideal gas in a closed 1L container this would result in a pressure of 700 atm according to. P V = n R T. From the phase diagram nitrogen is a gas at standard pressure and becomes supercritical at approximately 100 atm.
Phase diagram of nitrogen. diagrams for steels, the compound layer growth model is proposed to simulate the gas nitriding process of steels. By using this model, the properties of the nitrided steels based on the phase constitution, surface nitrogen concentration, nitrogen concentration profile, Nitrogen phase diagram pressure temperature. The curve between the critical point and the triple point shows the nitrogen boiling point with changes in pressure. I know that phase diagram says after critical temperature and pressure we have a supercritical phase but with enough p and t more exotic states of matter occur. Solid nitrogen melts at ... Nitrogen. Formula: N 2. Molecular weight: 28.0134. IUPAC Standard InChI: InChI=1S/N2/c1-2. Copy Sheet of paper on top of another sheet. IUPAC Standard InChIKey: IJGRMHOSHXDMSA-UHFFFAOYSA-N. Copy Sheet of paper on top of another sheet. CAS Registry Number: 7727-37-9. File:Phase diagram of nitrogen (1975).png ... English: These 1975 phase diagrams are generally incomplete, reaching at most 250 kbar (25 ...
Nitrogen - Density and Specific Weight Online calculator, figures and tables showing density and specific weight of nitrogen, N 2, at temperatures ranging from -175 to 1325 °C (-280 to 2400 °F) at atmospheric and higher pressure - Imperial and SI Units Phase diagram. Detailed enthalpy calculations for the most stable structures allowed us to reconstruct the pressure-composition phase diagram ().The first thermodynamically stable carbon nitride ... To accurately define important phase boundaries in the iron-nitrogen (temperature-composition) phase diagram as well as the (temperature-potential) Lehrer diagram, the solubility of nitrogen in ferrite was determined as a function of the nitriding potential (which defines the chemical potential of nitrogen) and the temperature. To this end, thin iron foils were homogeneously nitrided in ... ADVERTISEMENTS: There is 78% nitrogen in atmosphere. But this cannot be taken in by the organisms. It needs to be fixed as nitrates and then utilised. This cycle is divided into four phases - nitrogen fixation, ammonification, nitrification, and denitrification (Fig. 12). Phase # 1. Nitrogen Fixation: Fixation of nitrogen takes place by atmospheric and […]
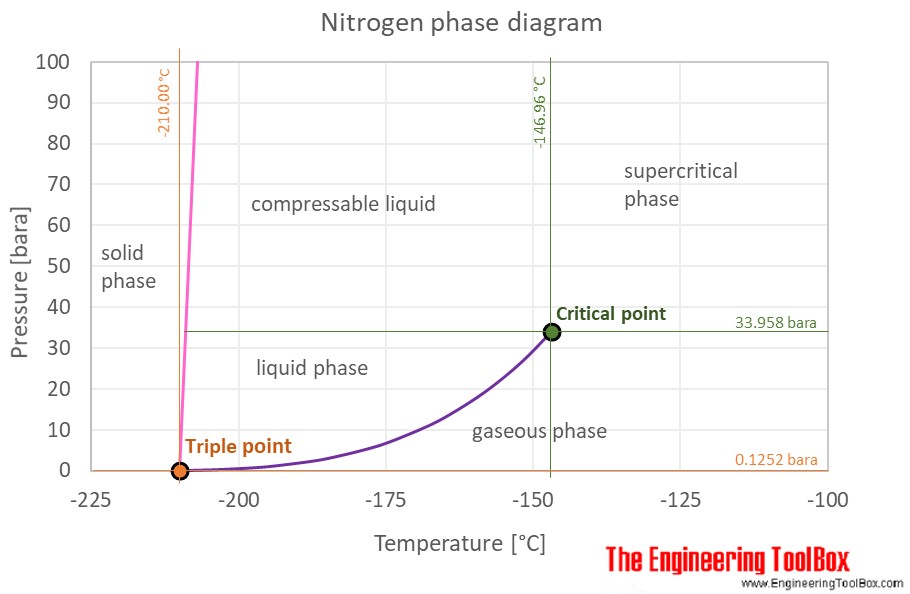
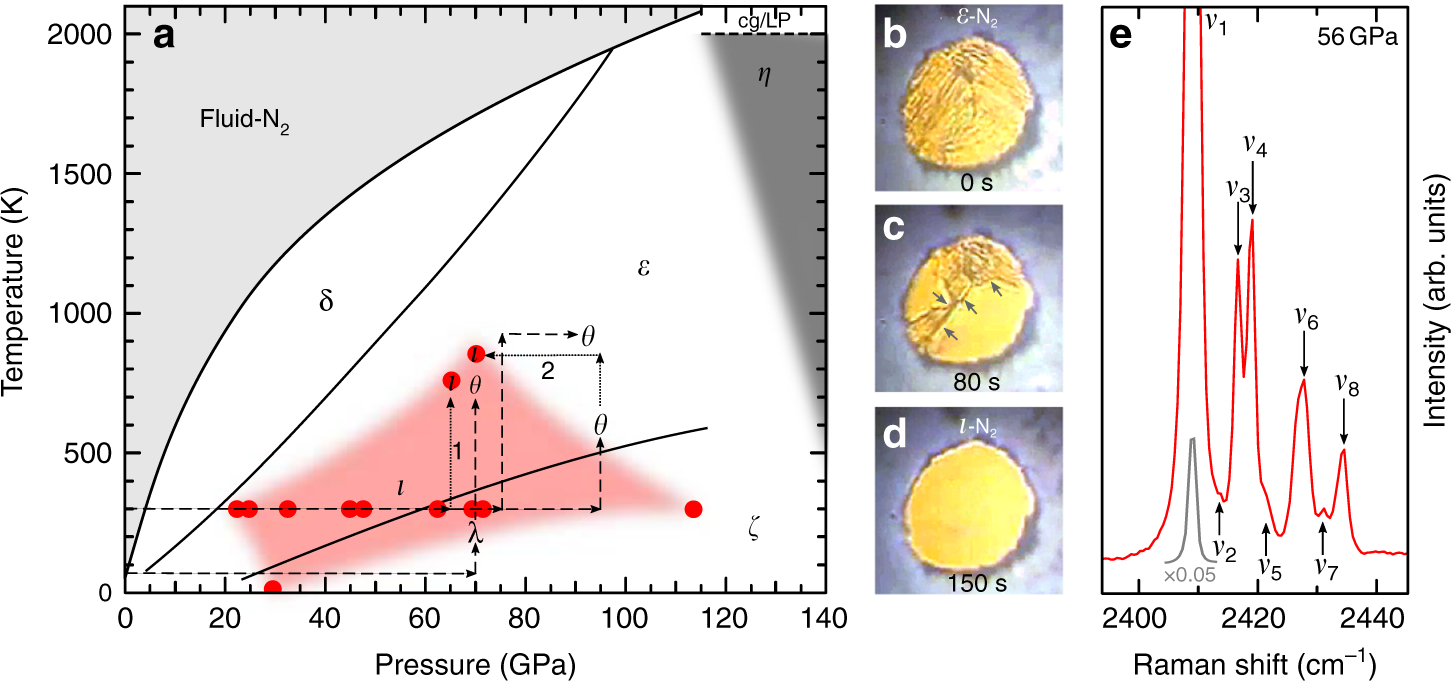
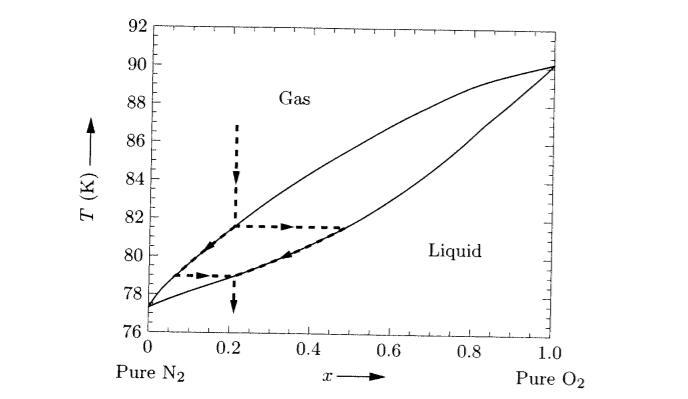
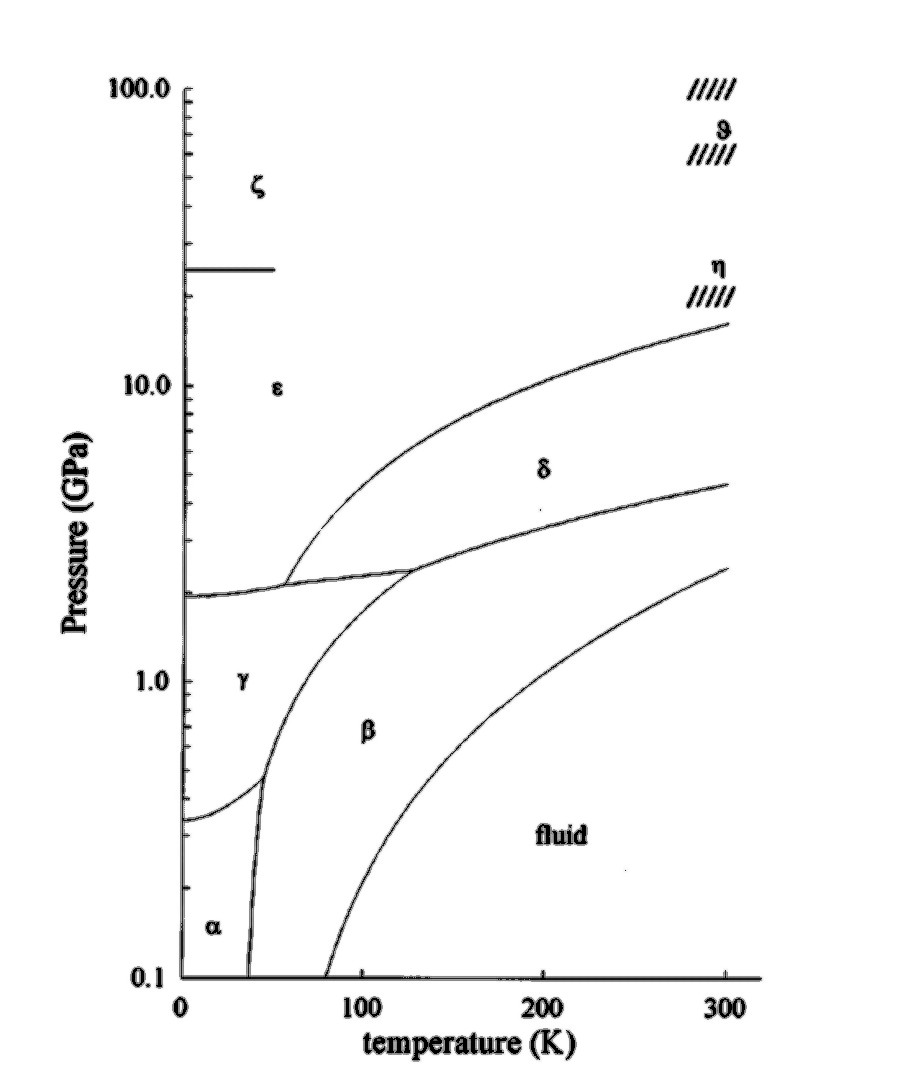
Procedure Looking at the isobars in the temperature-concentration phase diagram (schematically shown in Fig. 2) it can be seen that at constant nitrogen pres- sure the nitrogen concentration in solid molybdenum increases with rising temperature until the solidus line is reached and the sample melts because of the appearance of a liquid phase. ABSTRACT. We have determined the phase diagram for the notrogen-oxygen system by examining the x‐ray diffraction patterns of polycrystalline samples of the solidified mixtures over the temperature range 21°-50°K. The diagram exhibits a surprising complexity: a eutectic line divides the liquid and the two‐phase region of ‐ ‐ (γ ... Liquid nitrogen is very cold and and contact may cause frostbite. Under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. The phase diagram of nitrogen is shown below the table. Chemical, physical and thermal properties of Nitrogen: Values at 25 o C (77 o F, 298 K) and atmospheric pressure. Molecular Weight. The P-T phase diagrams in nitrogen as determined by Raman and X-ray scattering have been reported in the literature [2, 3, 10, 11, 18,19,20,21,22,23], as also given previously . Regarding polymerization and shock cooling, the high-pressure phase diagram of nitrogen has been given .
An experimental study of the phase transitions at high temperature in compressed solid nitrogen has been performed using Raman spectroscopy. Knowledge of the equilibrium phase diagram in the ...
Incorporating these ratios into the phase solidification diagram helps to predict whether the solidification of a Cr-Ni stainless steel occurs in primary ferritic or austenitic phase. This approach also helps users to understand how the increased nitrogen content in additively manufactured S174 results in the greater retention of austenite -
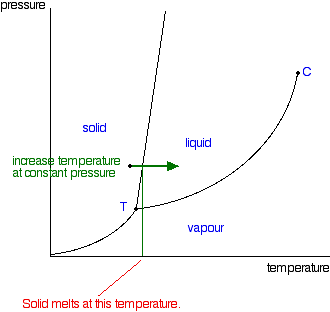
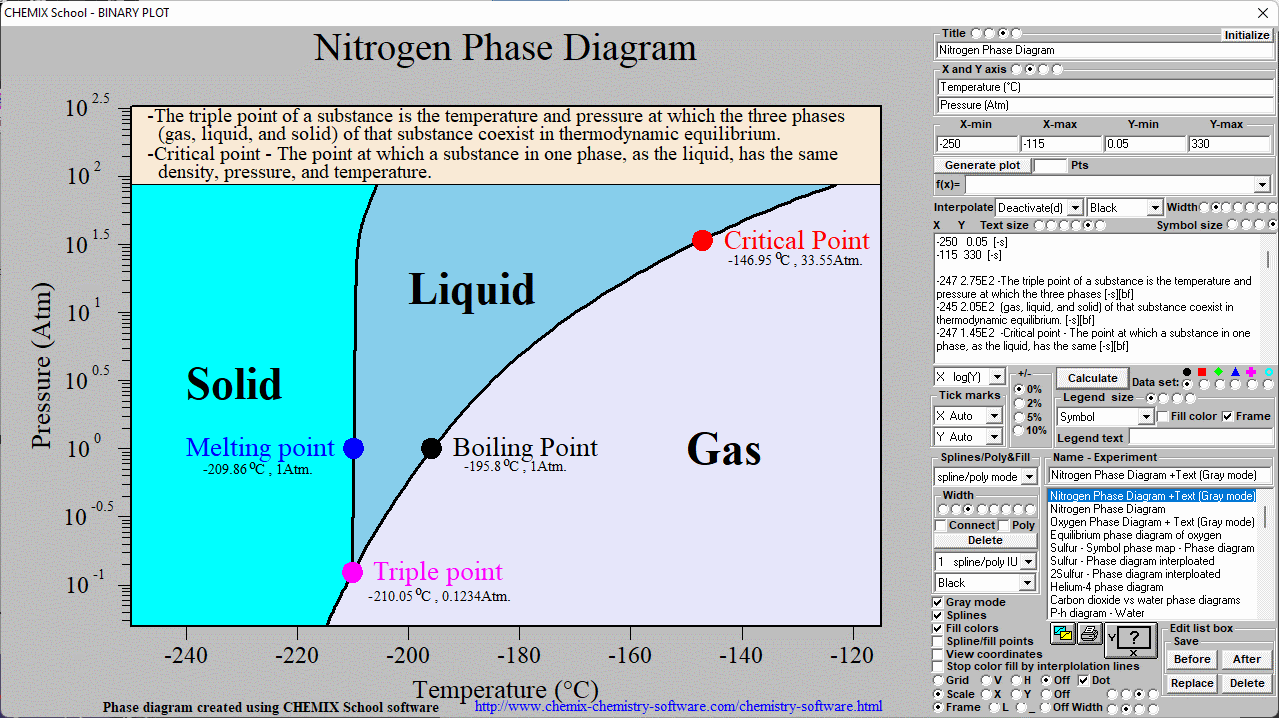
The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ...

Direct Gaseous Nitridation Of The Ti 6al 4v Alloy By Nitrogen Physical Chemistry Chemical Physics Rsc Publishing
Nitrogen phase diagram. Chemistry Software Download Melting point for Nitrogen ( 1 atm ) : 209.86 C Celsius , 63.29 K Kelvin , -345.75 F Fahrenheit

Thermodynamic Considerations Regarding The Ln2 In A High Pressure Freezer Science Lab Leica Microsystems
Phase Diagram Of Nitrogen. diagram liquid nitrogen phase diagram template information title liquid nitrogen phase diagram categories diagram ♦ publised friday january 27th 2017 08 43 53 am nitrogen phase diagram educational chemistry software chemistry software download melting point for nitrogen 1 atm 209 86 c celsius 63 29 k kelvin 345 75 f fahrenheit boiling point for

Scielo Brasil Prediction Of Phase Composition And Nitrogen Concentration During The Nitriding Process In Low Alloy Steel Prediction Of Phase Composition And Nitrogen Concentration During The Nitriding Process In Low Alloy Steel
32Ron: P. Röntgen and H. Braun, "On the Solubility of Gases in Metals: The Behavior of Hydrogen and Nitrogen Against Aluminum,"Metallwirtschaft, 11, 471-472 (1932) in German. (Equi Diagram; Experimental) Google Scholar
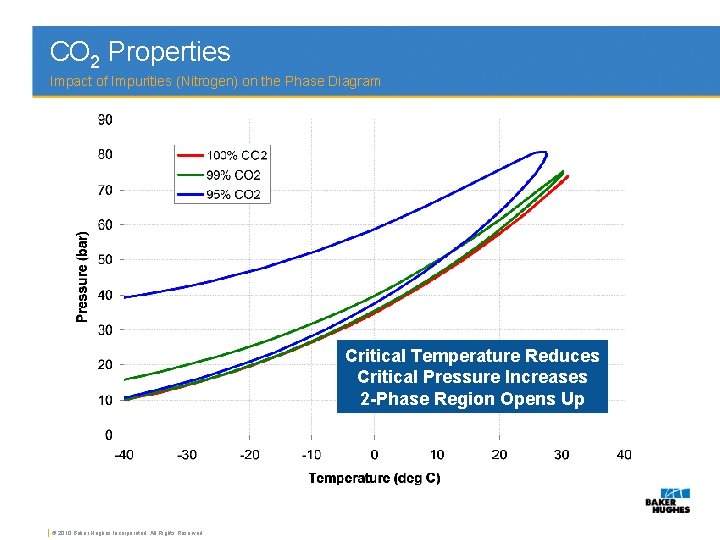
Phase diagrams of carbon dioxide, nitrogen and their mixtures with different amounts of nitrogen (e.g. 5 mol%, 10 mol% N. 2) were calculated with high accuracy with the NIST Reference Fluid Thermodynamic and Transport Properties database program REFPROP® for up to 200 bar, as well as density-pressure diagrams.
Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase.
Phase diagrams of carbon dioxide, nitrogen and their mixtures with altered amounts of nitrogen (e.g. 5 mol%, 10 mol N2) were affected with aerial accurateness with the NIST Reference Fluid Thermodynamic and Transport Properties database affairs REFPROP® for up to 200 bar, as able-bodied as..FactSage 8.1 - List of Stored Phase Diagrams (7811). FACT Databases.
the ε phase to form. As the temperature is further increased to the gamma prime (γ′) phase temperature at 490 °C (914 °F), the "window" or limit of solubility begins to decrease at a temperature of approximately 680 °C (1256 °F). The equilibrium diagram shows that control of the nitrogen dif-fusion is critical to process success ...
Nitrogen is a typical molecular solid with relatively weak van der Waals intermolecular interactions but strong intramolecular interaction arising from the second highest binding energy of all diatomic molecules. The phase diagram of solid nitrogen is, however, complicated at high pressures, as inter-molecular interaction becomes comparable to the intra-molecular interaction.
Calculation of the P–T phase diagram of nitrogen using a mean field model. International Journal of Modern Physics B 2017, ...
Thus we predict the solid-state phase diagram of boron-carbon-nitrogen (B-C-N). Owing to the large energy costs of substitution, we find that the mutual solubilities of the ultrahard materials diamond and cubic boron nitride are negligible, and the same for the quasi-two-dimensional materials graphite and hexagonal boron nitride.
nitrogen phase diagram - Wolfram|Alpha. Area of a circle? Easy as pi (e). Unlock Step-by-Step. nitrogen phase diagram. Natural Language. Math Input.
Download scientific diagram | The temperature-pressure phase diagram for nitrogen from publication: Selected aspects of manufacturing and strength ...
pressure phase diagrams. RESULTS On the Nitrogen Phase Diagram. We first apply the approach combining first-principles computations with ther-modynamic calculations to identify the phase boundary between molecular nitrogen and polymeric nitrogen. Triple-bonded molecular nitrogen is represented using the crystal structure of ε-N2 (SpGr. R3̅c ...
Figure A-14EP-h diagram for refrigerant-134a Table A-16EProperties of the atmosphere at high altitude Table A-17EIdeal-gas properties of air Table A-18EIdeal-gas properties of nitrogen, N 2 Table A-19EIdeal-gas properties of oxygen, O 2 Table A-20EIdeal-gas properties of carbon dioxide, CO 2 Table A-21EIdeal-gas properties of ...
From the densities of liquid nitrogen and nitrogen gas at standard pressure the volume ratio is about 1:700. For an ideal gas in a closed 1L container this would result in a pressure of 700 atm according to. P V = n R T. From the phase diagram nitrogen is a gas at standard pressure and becomes supercritical at approximately 100 atm.
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
Solved Liquid Nitrogen And Carbon Dioxide Can Be Used As A Refrigerant In Many Food Freezing Applications You Are Assigned By The Project Manager Course Hero
C. Boron carbon nitrogen ternary Little experimental data exist on the B-C-N ternary. Previous phase diagrams are either presented at very high temperature or lack detailed information, especially at the high boron corner [33]. No stable compounds have been reported in the B-C-N ternary system. However, variants of diamond/c-BN such as BC 2N and BC
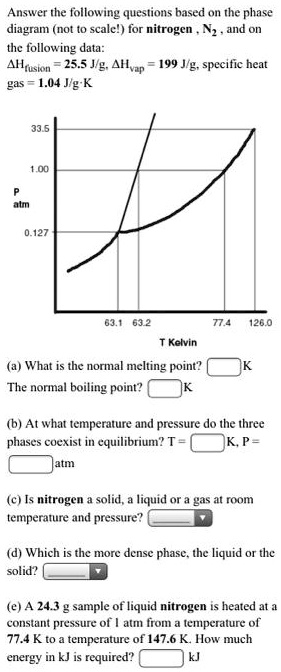
Solved Answerthe Following Questions Based On The Phase Diagram Not Scalel For Nitrogen And On The Following Data Ahuskon 255g Slvzr 199 J G Specific Heat G1s 1 04 Jlg K 0 127 03 1










0 Response to "35 phase diagram of nitrogen"
Post a Comment