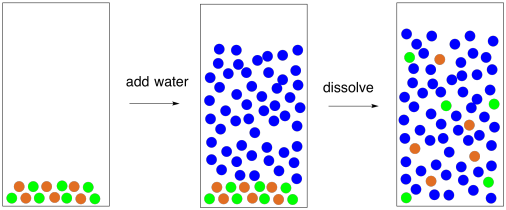
36 diagram of salt dissolved in water
A lot of people have posted theories on How the wall remains intact after thousands of years, with varying degrees of complexity and magic. I’ve always thought the answer seemed fairly simple, and other theories seemed needlessly complicated, so maybe it’s not obvious to everyone else, or perhaps I’m just missing something. If it’s just that the theory has already been posted to death and people want to come up with more interesting things, than I’m sorry for re-posting. The problem with the wa... Reverse Osmosis works by using a high pressure pump to increase the pressure on the salt side of the RO and force the water across the semi-permeable RO membrane, leaving almost all (around 95% to 99%) of dissolved salts behind in the reject stream. The amount of pressure required depends on the salt concentration of the feed water.
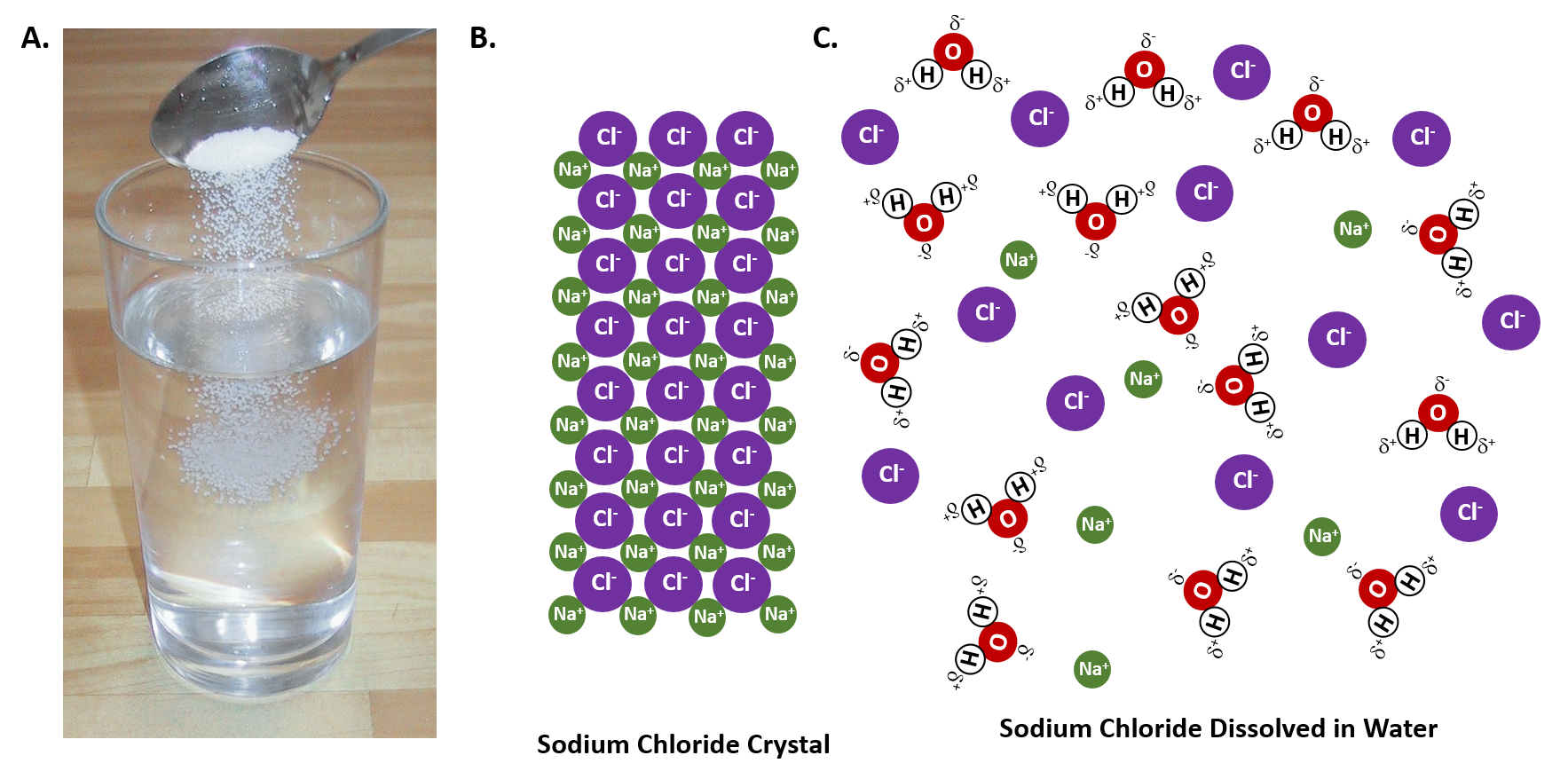
"Salt dissolved in water" is a rough description of Earth's oceans. In chemistry, it results in a solution, as the ionic bond of NaCl is pulled apart by the attraction of Na to the O of H2O and the Over two-thirds of Earth's surface is covered by ocean water, which is notably saline, or "salty," in character.
Diagram of salt dissolved in water
Oct 12, 2019 · The water filtration process begins by pushing water through a pre-filter, also known as a sediment filter. The process removes dissolved solids (TDS) like salts from the water. It removes about 90-99% of the salts, leaving the solid waste behind in the reject stream. Post this; the booster pump works by building water pressure. I'd like to preface this by saying I do not encourage IV drug use and acknowledge I have been wholly reckless, this is definitely not an example of responsible drug use. Some details are a bit fuzzy as this trip was taken in May of 2019. At the time: 26F, 167cm, 80kg My partner at the time (A) had been gifted 1 dose IV-able DMT Fumerate, but didn't want to take it. I had always wanted to try it. It lay around for a while, maybe 4-6 months. Neither of us knew the size of the dose* and I did not... I posted previously, but some revisions have happened from all the input I got. Also added labels to the diagrams, made the parts list a bit cleaner, and tried to organize this better. Found those water tests too. Anyway, posting here to hopefully get some input/advice/reasons why I'm stupid. Just a couple notes about my mindset: This a definitely overkill for what I need, but I'm okay with that. This has become a bit of a hobby project by now. Total price is about $7,000 (breakdown is on the p...
Diagram of salt dissolved in water. So, if you are using 1 liter containers you can add 6 tsp of salt for a total of 36 g of salt (6 tsp x 6 g per tsp =36 g), fill the containers to the top with water (32 oz is approximately 1 liter or 1000 g), mix until all the salt is dissolved and you'll have an salt solution that is approximately 36 ppt. Total Dissolved Solids, also known as TDS, are inorganic compounds found in water, such as salts, heavy metals, and some traces of organic compounds dissolved in water. Excluding the organic matters that are sometimes naturally present in water and the environment... Triangular diagrams are used for representing three-component systems. Every possible composition of the ternary mixture corresponds to a point in It changes the general properties or characteristics of the solvent. As soon as any salt dissolves in the water, the boiling point of the water gets affected... So I need someone to check some of these, so I might crosspost this to other subreddits, if you know any, please do. Or if you are an expert yourself, please correct me if there's any mistakes. But I did watch Dr. Stone in an *Anime Streaming website*, I posted some interesting comments in the discussions of Dr. Stone Episodes. I will post them in a chronological order with the matching episodes. Although, I think it's a bad idea to post this in a whole one post. Because no one gonna read it t...
Total Dissolved Solids. A water molecule in its purest form contains 2 atoms of Hydrogen and 1 atom of Oxygen. But, the water available from natural This is because water contains a lot of organic and inorganic salts dissolved in it. This water contains calcium, magnesium, sodium, potassium, sulfates... Total dissolved solids (TDS) is the term used to describe the inorganic salts and small amounts of organic matter present in solution in water. The principal constituents are usually calcium, magnesium, sodium, and potassium cations and carbonate, hydrogencarbonate, chloride, sulfate, and nitrate anions. Adding salt to water at ambient pressure affects its thermodynamic properties. At low salt concentration, anomalies such as the density maximum are shifted to lower Exploration of the phase diagram of liquid water in the low-temperature metastable region using synthetic fluid inclusions. When a non-volatile solute is dissolved in a pure solvent, its vapour pressure is decreased. These are known as Raoult's laws of elevation of the boiling point. So, the addition of salt will increases the boiling point of the solution.
Soo... I had a day off and I was anxious to get prime out of the way. These were a bit less inspired than some of the previous ones but at least I think I managed to avoid the usual quintessence manipulation rotes. Probably not the best sphere to focus on, but good enough for a secondary or third choice... The last rote was less about the players and more about story potential. It generates a temporary node, not a big deal, but the secrets within... So, any thoughts about this? A bit rushed, I ... • Solids dissolved in water: the salt-water and the sugar-water systems are chosen, as the most proximate to everybody's experience. 1. The interest is just on liquid solutions, since the components do not mix in the solid state, and the amount of salt vapours can be neglected below say 1000 ºC. The experiment is based on the cooling of a boiled salt water solution where the variable is the amount of salt. Our goal is to determine whether salt concentration affects the rate at which boiled water cools. We hypothesize that the higher the concentration of salt dissolved in a volume of water, the lower... However, salt in cold water does not dissolve as well as if the water is warm. They will pour half of the solution in another jar and place one end of the cotton string (mop string) in each of the two jars with Epsom salt solution, as shown in the diagram on the right.
Dissolved salts and gases in the raw water can be removed by aeration. Mixing water and air oxidizes these salts and makes them filterable. Carbon dioxide is also removed during aeration. The solubility of gas in water is directly proportional to its partial pressure in the surrounding atmosphere.
Hi all, I was recently listening to the glassware unboxing stream where it was mentioned that /u/ExplosionsAndFire wanted to make xenon trioxide. Before anything, I should mention I am NOT a professional chemist, and I have not attempted any of these reactions myself. I am simply going from my education (minor in chemistry) and the literature. Please ensure you look everything up and take safety precautions if you try this. I propose a slightly round-about method of production: First some war...
When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these C12H22O11 molecules are released into solution. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt.
Things like salt, sugar and coffee dissolve in water. They are soluble. They usually dissolve faster and better in warm or hot water. Two things that affect the speed at which a solid dissolves are temperature and the size of the grains of the solid. Caster sugar which is made of fine particles will...
Dear Cody, I am currently an honors chemistry student at Avon High School. We have just begun a project on Antifreeze. Our objective is to research the types of solutions and how those solutions are used in keeping cars cool without freezing and damaging the car. I obtained your contact information from your YouTube channel. Any information you can gives us about the different types of solutions, what they are made of, and how they work would be greatly appreciated. Also, I...
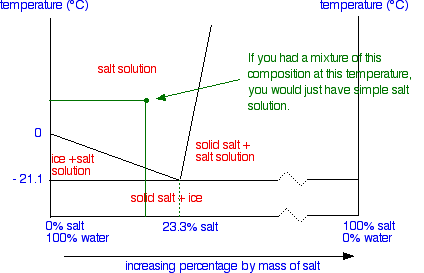
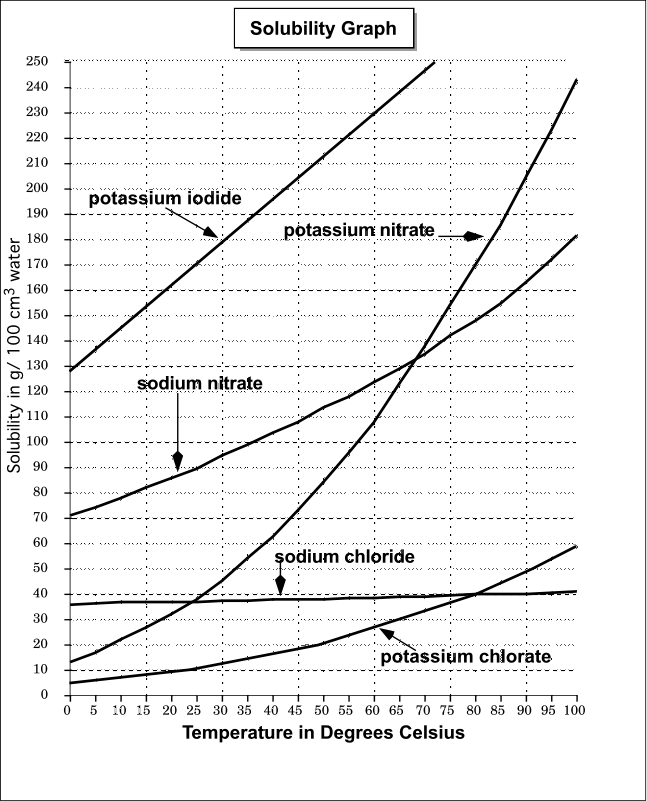
Shows how the phase diagram for mixtures of salt and water is built up, and how this leads to a eutectic A solution is saturated if it won't dissolve any more of the salt at that particular temperature - in the presence For example, suppose you have a near-boiling solution of potassium nitrate in water.
Short preface here: This whole post might get a little long, but it's something interesting for anyone looking for a neat and easy experiment. r/chemistry is home to people ranging from high school students to experienced PhD holders, but my goal with most material is to make it presentable to any audience. If you have taken some undergraduate chemistry/physics this experiment is inherently a lot more accessible due to an understanding of how light works (and you may be able to zoom past some be...
Warmer water will dissolve more salt than cooler water, regardless of the type of salt you are using. If you are doing a formal experiment, you should record The amount of salt you can dissolve in water depends on a few variables like temperature and purity. To dissolve salt into water, just stir it in with...
From what I understand, when table salt dissolves, the water molecules actually separate the salt molecules into the individual Ions Na+ and Cl How come the water molecules have enough of a magnetic force to separate the bonds of NaCl, but not strong enough to form new bonds with the Ions?
Saline water (more commonly known as salt water) is water that contains a high concentration of dissolved salts (mainly sodium chloride).The salt concentration is usually expressed in parts per thousand (permille, ‰) and parts per million (ppm). The United States Geological Survey classifies saline water in three salinity categories. Salt concentration in slightly saline water is around ...
Jun 28, 2017 · > Fresh water generator line diagram ... Increased solubility of CO2 generated from the salt water due to reduced sea water temperature. This dissolved CO2 makes water acidic and conductivity of water increases. Hence salinometer shows increased salinity, which is a measure of conductivity ans not presence of salt ...
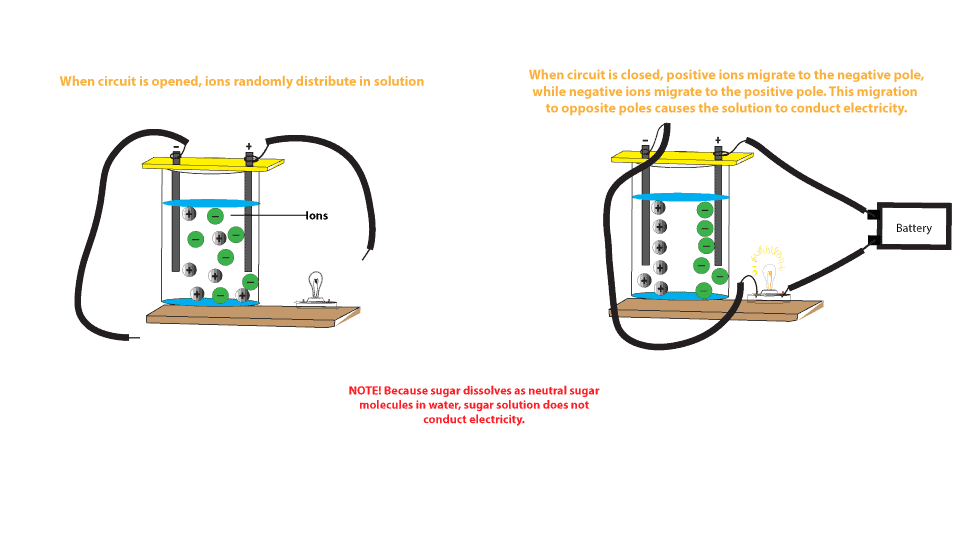
Salts dissolve in water to produce an anion and a cation. These ions make up the basis of conductivity in water. Conductivity is a measure of water's capability to pass electrical flow. This ability is directly related to the concentration of ions in the water 1. These conductive ions come from dissolved salts...
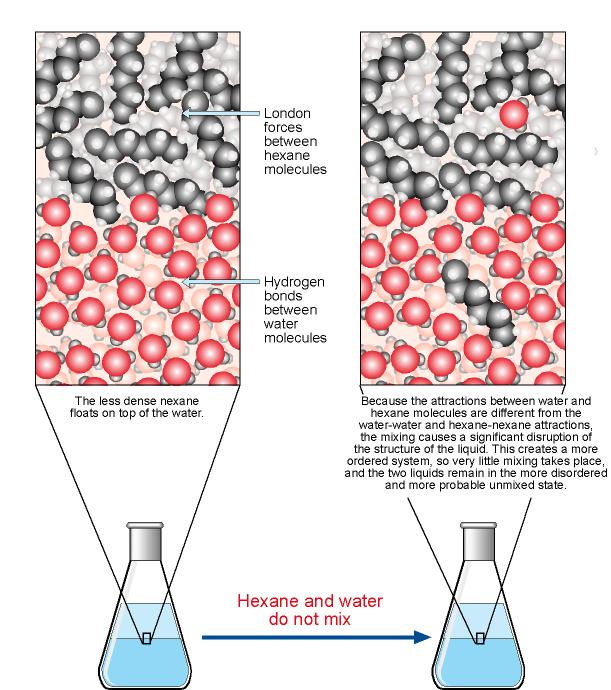
After seeing an animation of water dissolving salt, students will compare how well water and alcohol dissolve salt. They will relate their observations to the structure of salt, water, and alcohol on the molecular Another way of saying this is that the solubility of salt is greater in water than in alcohol.
It was their favorite prank. Isa Jones and Halshaa Vess were mini-celebrities at the University. Two Lords of War, from both species, both teaching history at a humble university many light-years away from their home. What had led them there was the source of unending rumors; they were political exiles, outlaws, scouts for eventual invasion, their "backstory" got more and more elaborate with each retelling. But when Isa and Halshaa both found out they were from The Deep, an idea formed betwe...
This list was abandoned and whoever originally posted it went and got their account deleted. Let's do it a favor and cast true resurrection! 1. An old but seemingly undamaged piece of abstract art containing a pattern designed to crash the viewer's brain. 2. A small box containing an AI brain, with no apparent means of interacting with the world other than a small speaker and an adapting USB like system. When picked up, it will immediately try to convince the PCs to load it into a more capable...
I'd like to preface this by saying I do not encourage IV drug use and acknowledge I have been wholly reckless, this is definitely not an example of responsible drug use. Some details are a bit fuzzy as this trip was taken in May of 2019. At the time: 26F, 167cm, 80kg My partner at the time (A) had been gifted 1 dose IV-able DMT Fumerate, but didn't want to take it. I had always wanted to try it. It lay around for a while, maybe 4-6 months. Neither of us knew the size of the dose* and I did not...
This list combines my favorite entries from [these](https://www.reddit.com/r/d100/comments/7ykf1b/lets_build_100_magic_weather_effects/) [two](https://www.reddit.com/r/d100/comments/7quk2l/lets_build_100_magical_storms/) weather threads. I modified some entries from their original sources. d100| Uh oh, the magic barometer is reading low. The forecast for the upcoming weather is...|Credit ---------|----------|---------- 1 | Daggerfall: It rains daggers dealing 1d4 damage / round people are out u...
Seriously guys... we need to talk about this. I see more and more posts regarding how soil is superior, how better it is, how "organic" it is, how much more "natural" it is then all these "synthetic chemical" bottled nutrients... [https://en.wikipedia.org/wiki/Soil#Composition](https://en.wikipedia.org/wiki/Soil#Composition) " A typical soil is about **50% solids (45% mineral and 5% organic matter)**, and 50% voids (or pores) of which half is occupied by water and half by gas. " ...
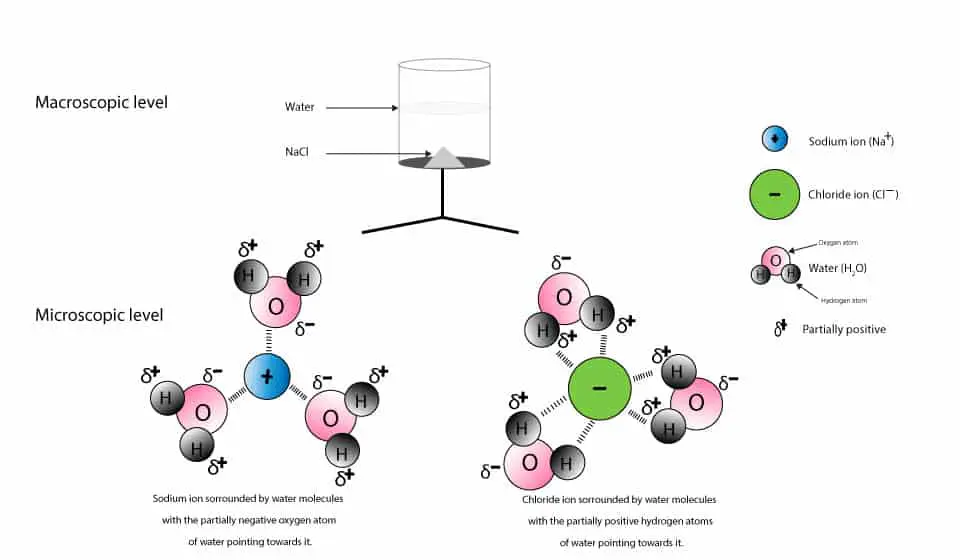
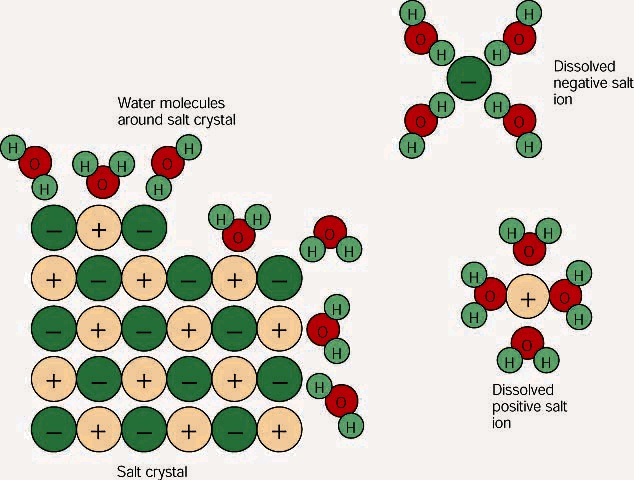
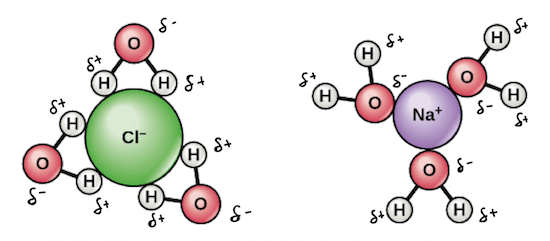
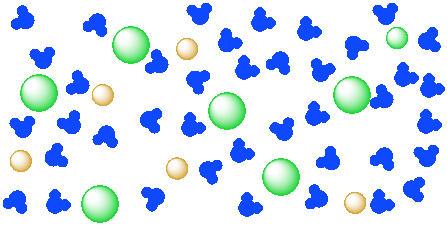
When salt dissolves in water, water molecules get interspersed between the sodium and chlorine atoms, which means that they don't interact significantly with the After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows.
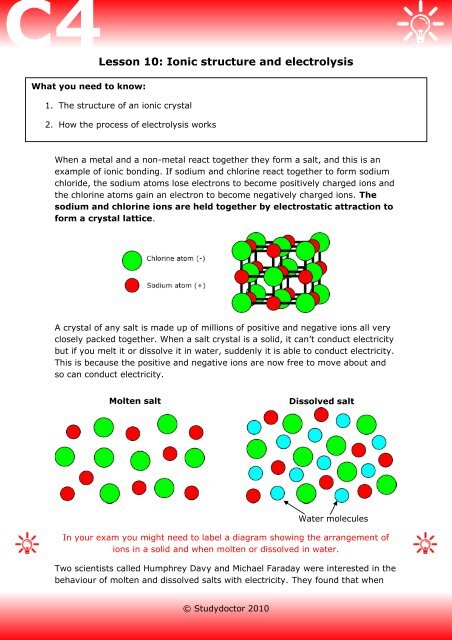
Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and Thus, any ionic compound that is soluble in water would experience a chemical change. In contrast, dissolving a covalent compound like sugar does...
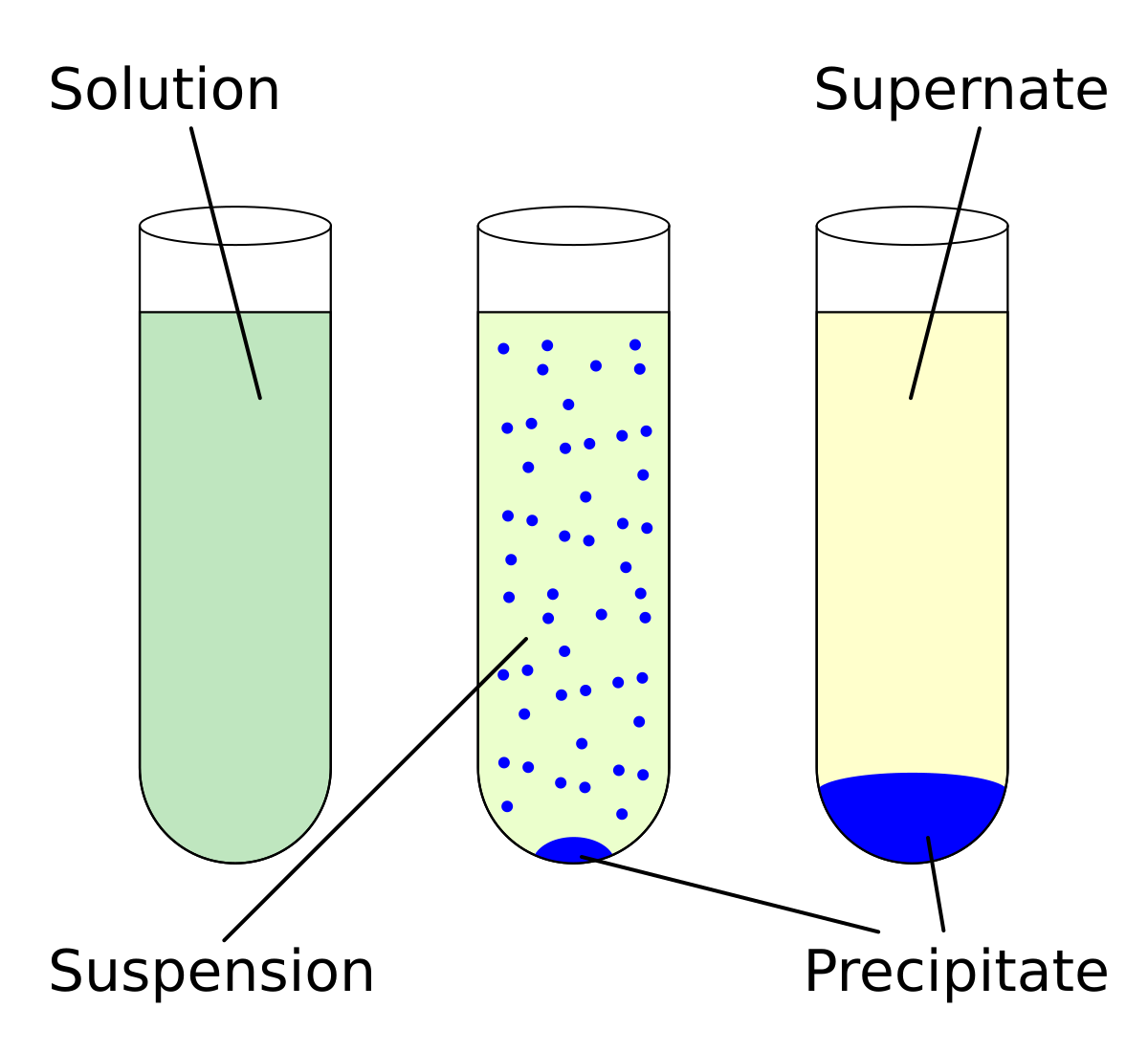
Salt water, for example, is a solution of salt (a solid) in water (a liquid). Rubbing alcohol is also a solution, where water is dissolved in isopropyl 2 Supersaturated solutions A supersaturated solution has more salt dissolved in it than theoretically possible. The formation of crystals is not always easy...

Medical Cell Biology Self Directed Learning Module Diffusion Across Membranes Osmosis Properties Of Ions Michael L Jennings Department Of Physiology And Biophysics September 24 2012 Preface You Have Undoubtedly Heard About Diffusion And
In the case of table salt mixed with water, Na and Cl atoms, initially bonded together in the form of a crystal, are dissolved by molecules of water. Learning goals. To illustrate the different stages in the dissolution of salt (sodium chloride) in water. To introduce electrostatic phenomena ( ionic charges...

Transparent Hydrogel With Enhanced Water Retention Capacity By Introducing Highly Hydratable Salt Applied Physics Letters Vol 105 No 15
I have finally decided to extend Plato's elementary system to only one, the other three remaining polyhedra will not be used until you find good options (if you have read my previous posts, you will understand what I mean, anyway this results in a very different from magic to the above, so you do not miss anything). **I explain:** I have been searching for a long time an extended system of classical elements (a system with 9 elements) but that will fit the system of elements of Plato and mediev...
Seawater, or salt water, is water from a sea or ocean.On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/l, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately 35 grams (1.2 oz) of dissolved salts (predominantly sodium (Na +) and chloride (Cl −) ions).Average density at the surface is 1.025 kg/l.
**PART TWO HUNDRED AND SEVENTY-TWO** ***Sunday*** Llyr took one look at Sam standing beside the bed with Geraldine wrapped around his neck and went into crisis mode. Nothing and no one in the room mattered more to him than his son. About the only reason Llyr didn’t break Clefton’s neck on his way through, was Clefton said, “Shit, I’m calling your old man…” not knowing that Llyr had arrived seconds earlier. “I’m already here,” he said, shoving Clefton aside. The girl had enough self-preservat...
This phase diagram shows the solubility of calcium sulfate in water at temperatures between 0 and 200 °C. Gypsum and The reason why the solubility increases is that the aqueous ionic species take up less space than the solid salt. The salt therefore prefers to be dissolved at high pressure.
Ocean water is about 3.5% salt. That means that if the oceans dried up completely, enough salt would be left behind to build a 180-mile-tall, one About 90 percent of that salt would be sodium chloride, or ordinary table salt. Chlorine, sodium and the other major dissolved salts of the ocean are listed in...
Dissociation of salt (sodium chloride) in water creating sodium chloride solution.
Artificial lighting means any source of light that does not come from the sun. Natural lighting is defined light that comes from the sun or other stars. Traditionally, artificial light comes from torches, oil lamps, or magical lamps. Generally, none of these involve any living organisms, and rely on combustion. However, there are alternatives to this method of classification, and they rely on something else: living organisms. In order to describe them, we’re going to have a new, ad-hoc category:...
Salinity is the saltiness or measure of dissolved salt in water. 35 g dissolved salt / kg sea water = 35 ppt = 35 o/oo = 3.5% = 35000 ppm = 35000 mg/l. fresh water (typical city water in United States) : < 100 ppm. water supply typicaly restricted to : 500 ppm.
The water coming out of these vents is full of dissolved minerals, even salt; this is one way that the oceans become salty. By the way, even miles below the surface, the areas around these vents are much warmer and support all kinds of unique ocean life that do not exist in other parts of the ocean.
hi guys imma just type the summary of things we need to know for chem o lvl's tmr (pls add on in the comments if i miss out something so i can edit this again) in hoping to help out others while also helping me to revise bcos typing notes is wayy faster than writing :) also this wld not be super precise bcos im just typing in the summary if not i'm literally typing a whole textbook here, but i'll try my best to put in all the infos that is important \*totally not last minute\* **kinetic partic...
Jun 06, 2019 · In this case, the concentration is the amount (by weight) of salt in water, as expressed in "parts per million" (ppm). If water has a concentration of 10,000 ppm of dissolved salts, then one percent of the weight of the water comes from dissolved salts. Here are our parameters for saline water: Freshwater - Less than 1,000 ppm
What happens when salt is dissolved in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows.
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. Find out more. Adhesion/cohesion
So [here](https://youtu.be/sxlJAkuGeM4) is the video I would love to have a conversation about. I wrote up a shortish analysis It was curious if anyone could help me continue the conversation not with myself haha. Analysis below. I absolutely love the imagery/symbolism of the child being the gateway to the cosmos and it's placement in the grotto. While being fed it's life force (water) from below the feet of the sun where the duality of man/nature (light and dark)(Masculinity/Femininity)(mi...



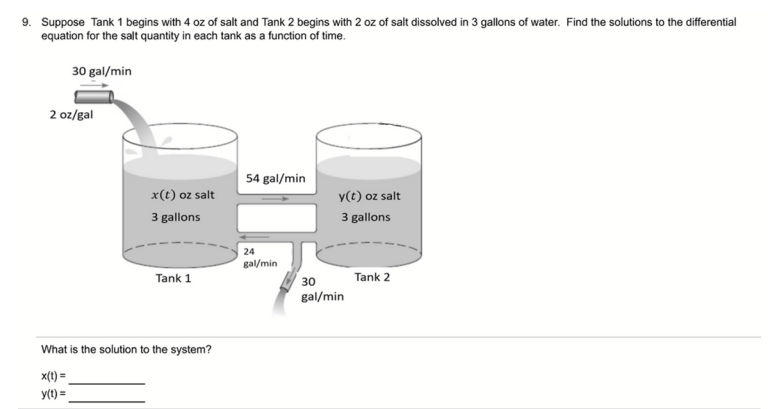












0 Response to "36 diagram of salt dissolved in water"
Post a Comment