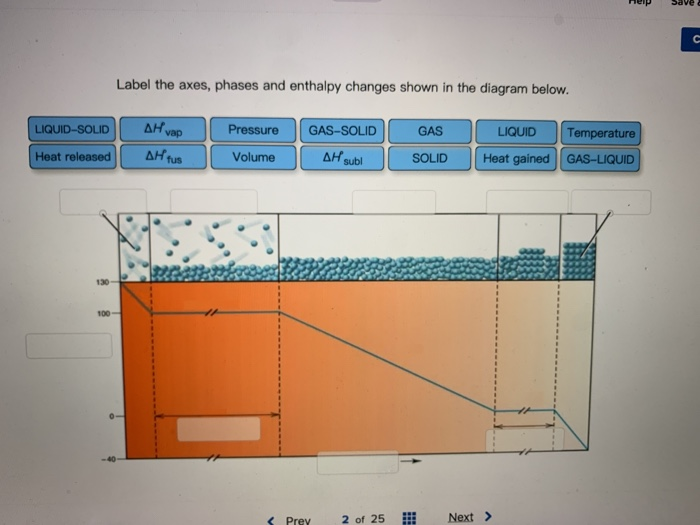
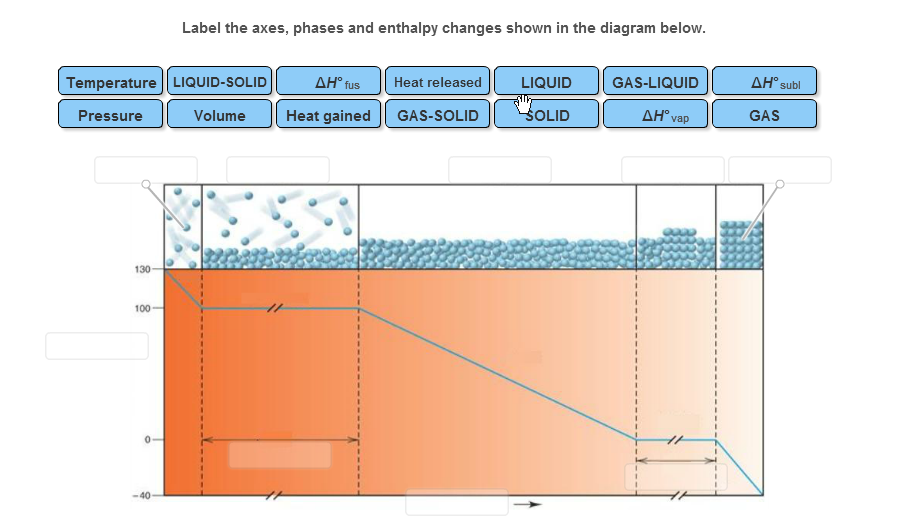
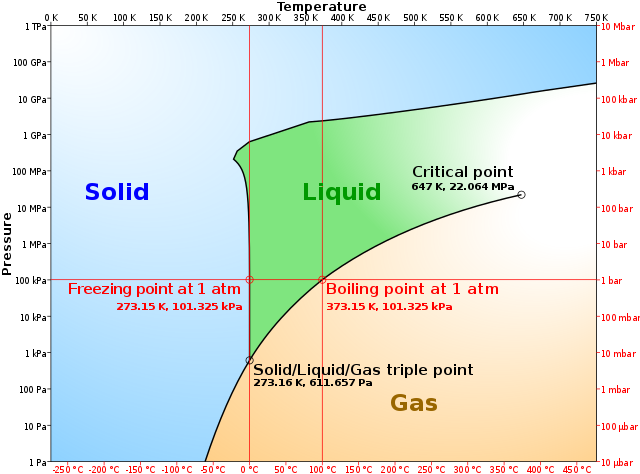
36 label the axes, phases and enthalpy changes shown in the diagram below.
gas Z as shown in the equation below. 2W(g)€ +€ Y(s)€ : € Z(g) The graph below shows how the concentration of Z varied with time at constant temperature. (i)€€€€€ On the axes above, sketch a curve to show how the concentration of W would change with time in the same experiment. Label this curve W. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. c) Calculate the energy difference between the reactants and the products. d) Deduce the sign of the enthalpy change. e) Identify with a reason, which is more stable, the reactants of products. 8. (N04/S/2)
The relative temperature changes of the copper and the water cannot be determined without knowing Tl and T2. 12(s) —Y HI(g) AH=26kJ/m01 HI(g) All=-5.OkJ/m01 64. Based on the information above, what is the enthalpy change for the sublimation of iodine, reprcsentcd below? (A) 15 kJ/m01rx,t (B) 21 kJ/molrxn (C) 31 kJ/m01rxn (D) 42 kJ/molrxn 62
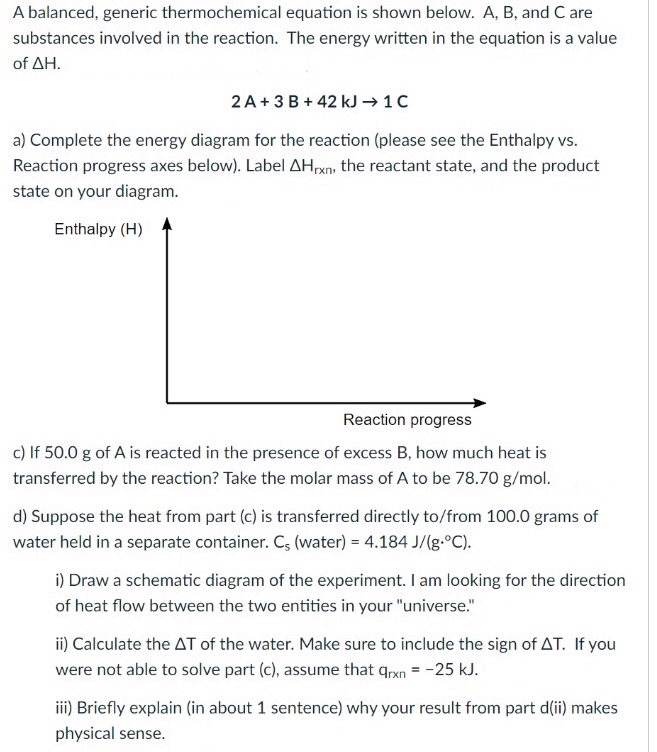
Label the axes, phases and enthalpy changes shown in the diagram below.
3.3 Phase Diagram for Water Vapor: Clausius-Clapeyron Equation. The Clausius-Clapeyron Equation. We can derive the equation for e s using two concepts you may have heard of and will learn about later: entropy and Gibbs free energy, which we will not go into here.Instead, we will quote the result, which is called the Clausius-Clapeyron Equation, Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. 1.18 DETERMINE the change in the enthalpy of a fluid as it passes through a system component, given the state of the fluid at the inlet and outlet of the component and either steam tables or a Mollier diagram.
Label the axes, phases and enthalpy changes shown in the diagram below.. appropriate label for the x-axis and y-axis, E a ... a reaction occurs as shown below. 3Fe(s) ... Calculate the activation energy for the forward reaction. b. Draw a labelled potential energy diagram showing the enthalpy change, and the activation energies for the forward and reverse reactions. What Is Required? Heat is added to the cylinder while the pressure is maintained constant until the temperature reaches 300°C, as shown in the following T-v diagram (temperature vs specific volume): From State (1) to State (2) the water maintains its liquid phase and the specific volume increases very slightly until the temperature reaches close to 100°C ... We will consider a phase change of 1 kg of liquid water contained within a piston-cycinder assembly as shown in Figure 3.2-1a. The water is at 20oC and 1.014 bar (or 1 atm) as indicated by point (1) on Figure 3.2-2. Figure 3.2-1 Phase change at constant pressure for water3 Figure 3.2-2 Sketch of T-v diagram for water4 Label the Axes, Phases, Phase Changes and Important Points On the Phase Diagram Below. the label gretty rose, label the goldfish, the label ritu kumar, the label network, label the heart game, label the parts of a flower, label the skull, label the x and y axis, label the food worksheet, label johnnie walker, 12 Label The Axes, Phases, Phase ...
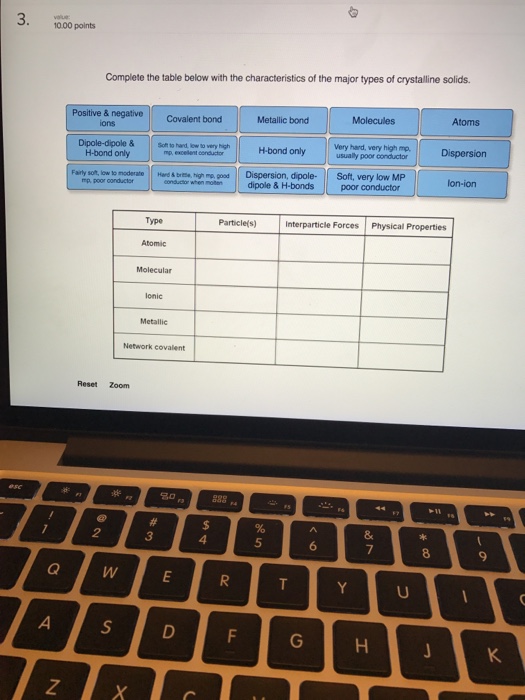
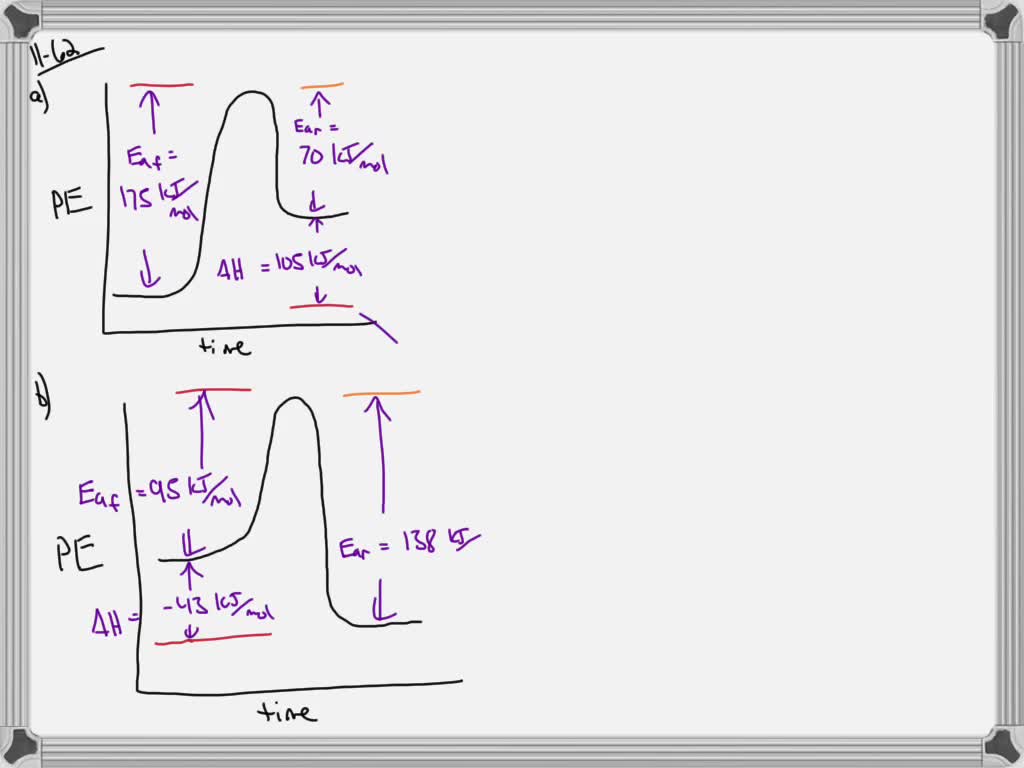
(c) The following enthalpy changes are given. enthalpy change value / kJ mol-1 standard enthalpy change of formation, , for K 3PO 4(s) -2035 standard enthalpy change, -ΔH o, for P(s) + 2O 2(g) + 3e PO 4 3-(aq) -1284 standard enthalpy change, ΔH +o, for K(s) K(aq) + e- -251 Determine the standard enthalpy change of solution of ... a) Draw a potential energy diagram for this reversible reaction. Your starting value for the reactants might be different that is ok, as long as you show the proper E a and ∆H values. b) Calculate the enthalpy change (∆H) for each reaction. i) ΔH forward = 137 kJ/mol Shown is the phase diagram for phosphorus a indicate the phases present in the regions labeled with a question mark b a sample of solid red phosphorus cannot be melted by heating in a container open to the atmosphere. 1000 points label the axes phases phase changes and important points on the phase diagram below. Label the axes, phases and enthalpy changes shown in the diagram below. Consider the reaction 3Fe(s) + 4H2O(g) ⇌ 4H2(g) + Fe3O4(s) If the total pressure is increased by reducing the volume, no change occurs.
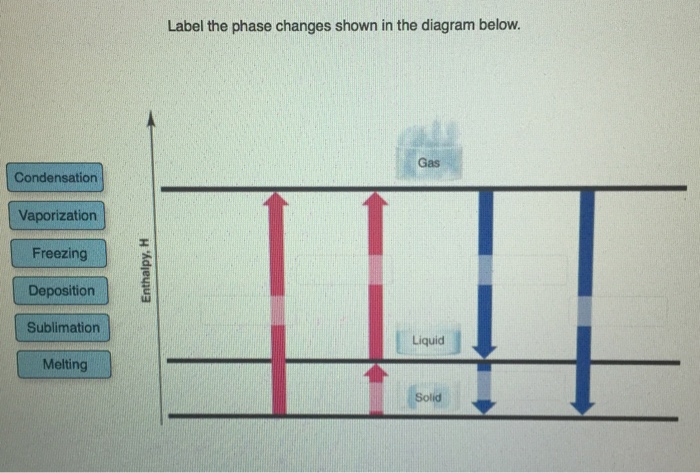
, as shown below: Now, let us remove the imaginary partition and let the A and B atoms mix. There should be some change in g due to this mixing, and it is given by ∆g. mix = ∆h. mix - T∆s. mix (3) The enthalpy term, ∆h. mix, represents the nature of the chemical bonding, or, put in Label the enthalpy changes shown in the diagram below. 32 examine the heating curve for water in section 117 figure 1136. 1000 points label the axes phases phase changes and important points on the phase diagram below. Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Label the phase changes shown in the diagram below. Gas Condensation Vaporization Freezing Deposition Sublimation Melting Liquid Solid. Label the enthalpy changes shown in the diagram below. 3. Label the axes, phases, phase changes and important points on the phase diagram below. 4. Label the axes, phases and enthalpy changes shown in the diagram below. Share this link with a friend: Copied! Other Related Materials.
label the axes phases and enthalpy changes shown in the diagram below › ... label the phase changes shown in the diagram below. reset zoom. 32 Label The Phase Changes Shown In The Diagram Below Written By Robert N Greenawalt Monday, August 9, 2021 1 Comment Edit.
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Label the axes, phases and enthalpy changes shown in the diagram below.
Shown is the phase diagram for phosphorus a indicate the phases present in the regions labeled with a question mark b a sample of solid red phosphorus cannot be melted by heating in a container open to the atmosphere. 1000 points label the axes phases phase changes and important points on the phase diagram below.
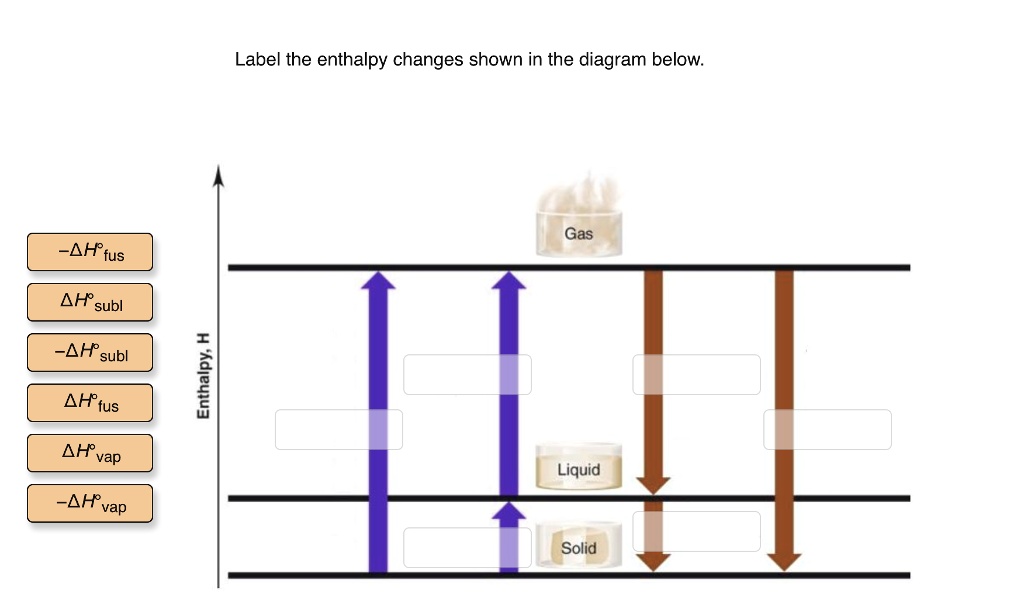
Solved Label The Enthalpy Changes Shown In The Diagram Below Gas Ah Fus Ah Subl Ah Subl Ah Fus Ah Vap Ah Vap Liquid Solid
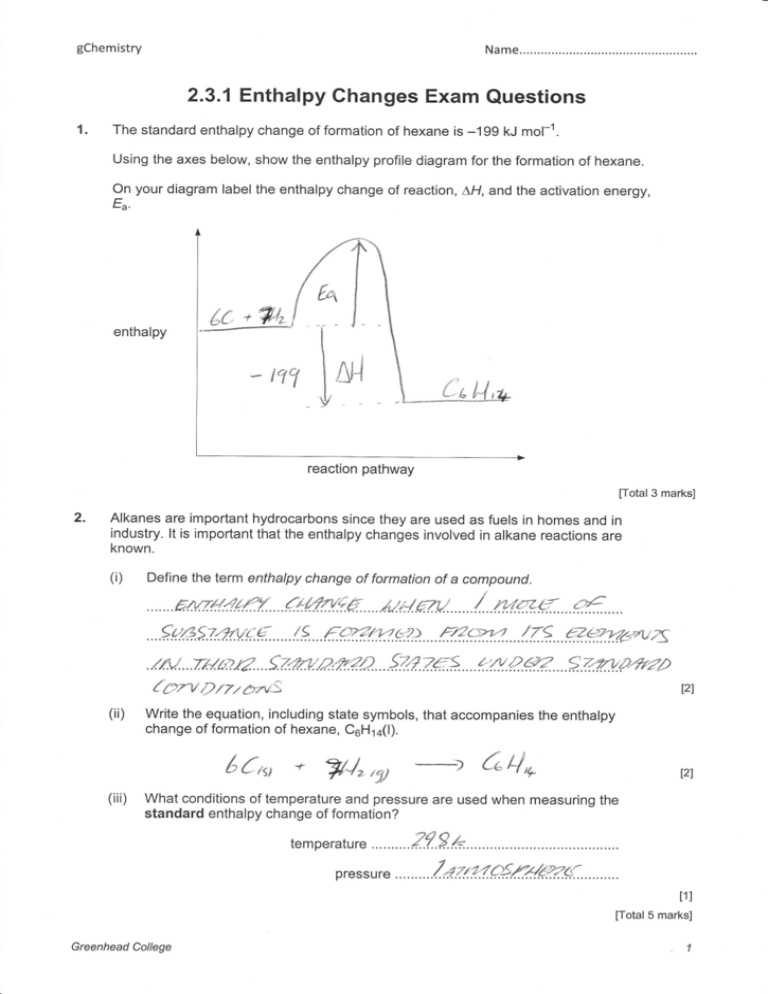
The standard enthalpy change of formation of hexane is -199 kJ mol -1. Using the axes below, show the enthalpy profile diagram for the formation of hexane. On your diagram label the enthalpy change of reaction, ∆H, and the activation energy, Ea. enthalpy reaction pathway [Total 3 marks] 17.
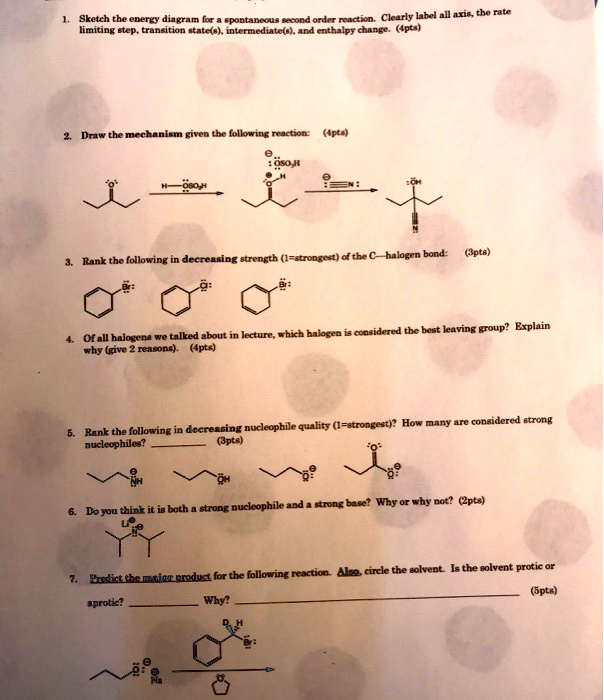
Solved Sketch Che Enerpy Uknm Clearly Label All Uris The Rate Pontaneou Erund Orc Nadton Lmltng Elod Traneition Tatc S Intcrmediatcle 4nd Cuthalpy Change Ipla Draw The Mechaniem Given The Following Reaction 4pta
Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. The phase diagram for xenon is shown belowin what. A typical phase diagram has pressure on the y axis and temperature on the x axis. Label the enthalpy changes shown in the diagram below.
Examining the Diagram. Let's look at the elements of this enthalpy diagram. First, as noted, the y-axis is labeled 'enthalpy' and the x-axis is labeled 'reaction progress.'Then we have the actual ...
The figure below shows a calculated phase diagram for the system H 2 O-Cu-NaOH-HCl-H 2 in which the X-axis is the molar ratio NaOH/(NaOH+HCl) (which is related to the pH) and the Y-axis is the equilibrium partial pressure of H 2 (which is related to the redox potential Eh).
(i)€€€€€€The diagram below shows the energy level diagram for the complete combustion of methane. Draw and label arrows on the diagram to show: •€€€€€€€€the activation energy •€€€€€€€€the enthalpy change, û+ . € (2)
It is interesting to note that the value observed for the phase change solid-liquid of C is comparable to the enthalpy of combustion of petroleum, which is 33,000 kJ/L (Diekmann et al., 1997), and to that of methanol, which is 22,700 kJ/kg (726 kJ/mol at 32 g/mol according to Atkins (1990)).
(a) Complete the enthalpy profile diagram below for the forward reaction in equilibrium 3.1. On your diagram: † Label the activation energy, E a † Label the enthalpy change of reaction, ΔH † Include the formulae of the reactants and products. enthalpy progress of reaction [2] (b) Calculate the activation energy, E a, for the reverse ...
Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase.
1.18 DETERMINE the change in the enthalpy of a fluid as it passes through a system component, given the state of the fluid at the inlet and outlet of the component and either steam tables or a Mollier diagram.
Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase.
3.3 Phase Diagram for Water Vapor: Clausius-Clapeyron Equation. The Clausius-Clapeyron Equation. We can derive the equation for e s using two concepts you may have heard of and will learn about later: entropy and Gibbs free energy, which we will not go into here.Instead, we will quote the result, which is called the Clausius-Clapeyron Equation,
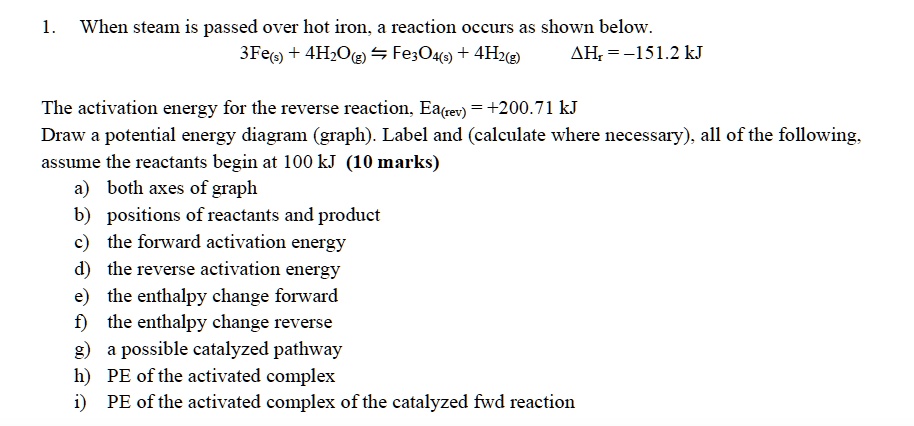
Solved When Steam Is Passed Over Hot Iron Reaction Occurs As Shown Below 3fe 4h Ok Fe3o4ts 4h2 Ah 151 2kj The Activation Energy For The Reverse Reaction Eatrev 200 71 Kj Draw Potential
Solved Consider The Following Reaction X Y 2 In Which Ah 340 0 Kj Sketch The Enthalpy Diagram For This Reaction Making Sure To Label Both Axes Reactant Product And Ah Is
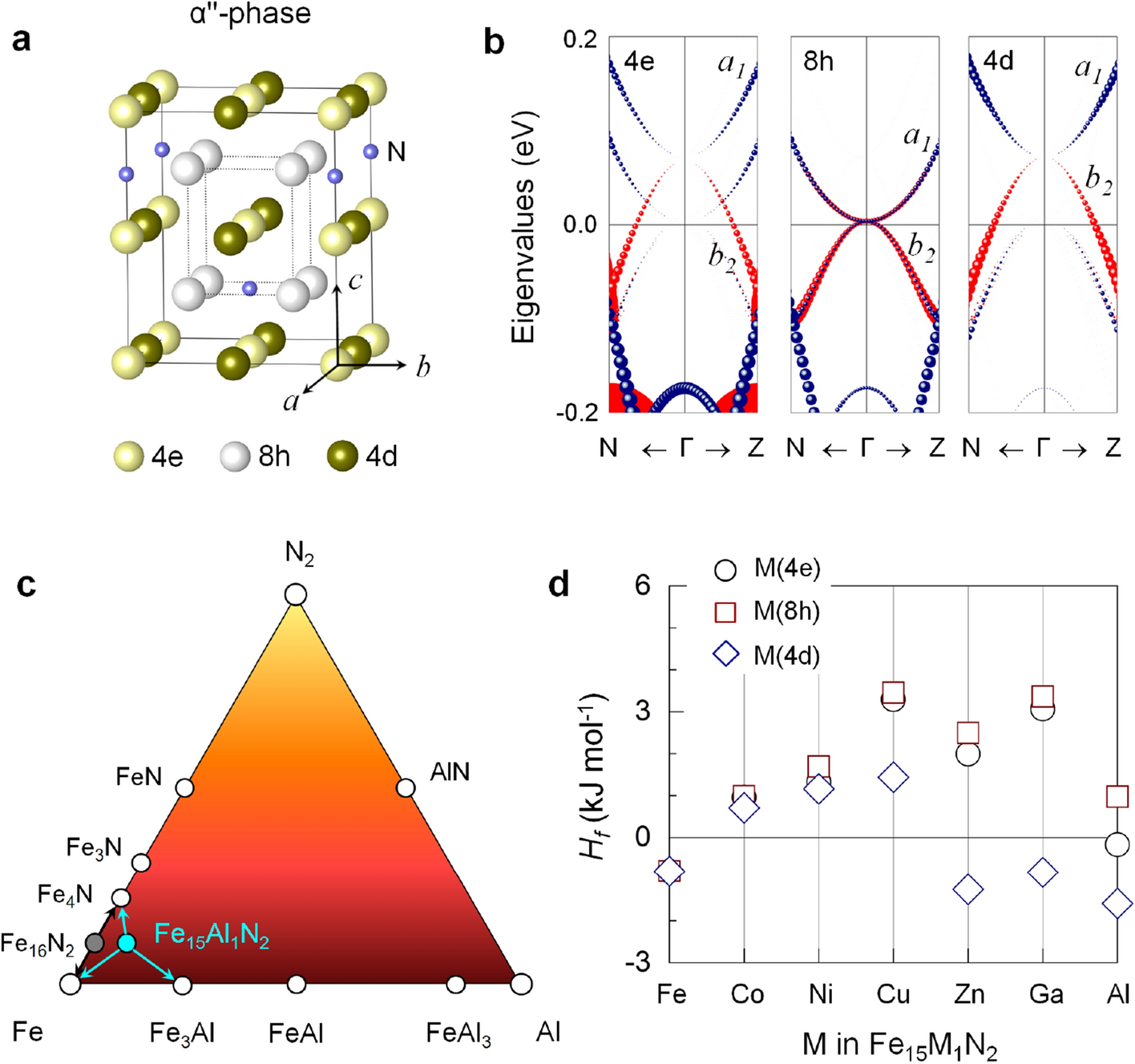
Simultaneous Tuning Of The Magnetic Anisotropy And Thermal Stability Of Alpha A Phase Fe 16 16 N 2 2 Scientific Reports
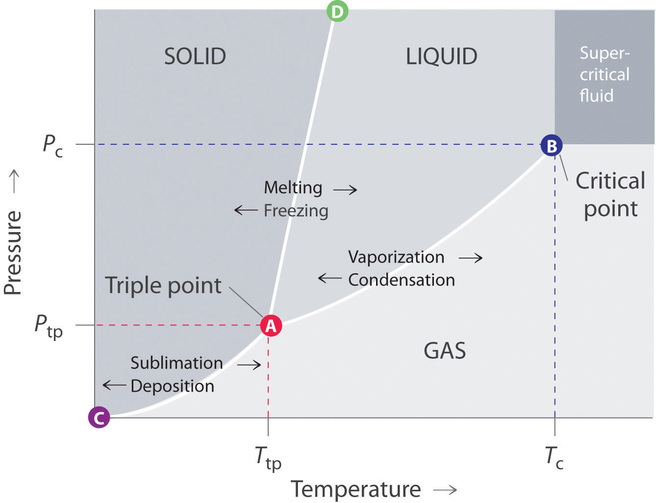
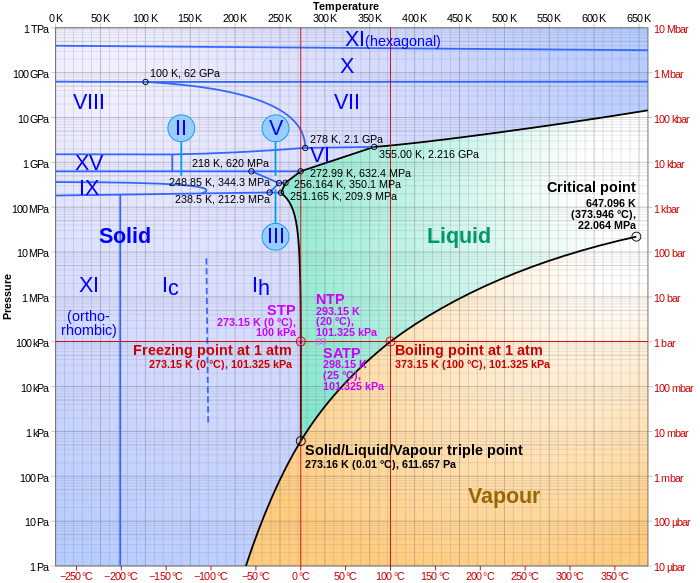
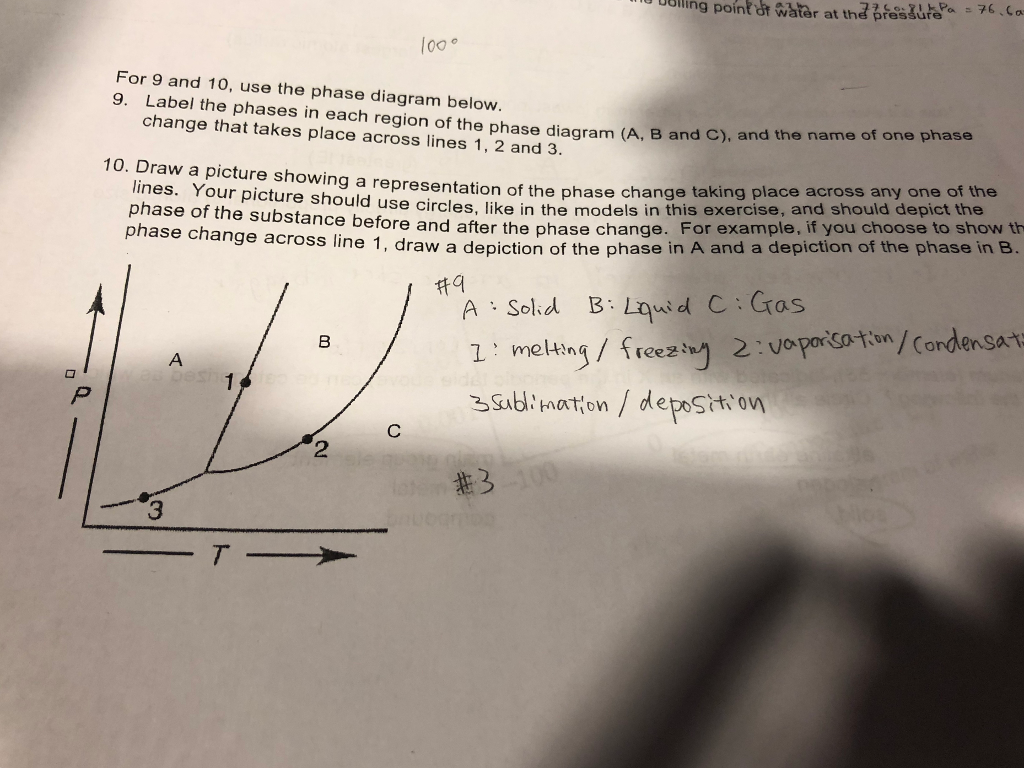

:max_bytes(150000):strip_icc()/phase_diagram_generic-56a12a1b5f9b58b7d0bca817.png)
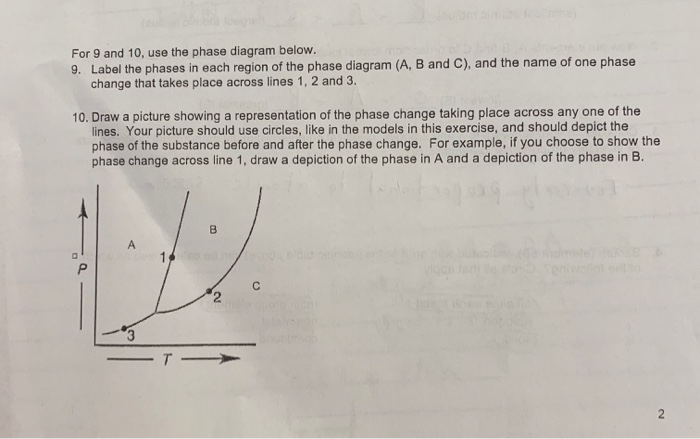








0 Response to "36 label the axes, phases and enthalpy changes shown in the diagram below."
Post a Comment