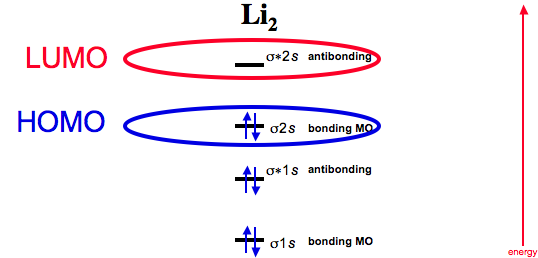
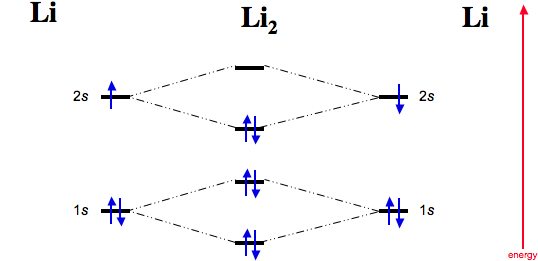
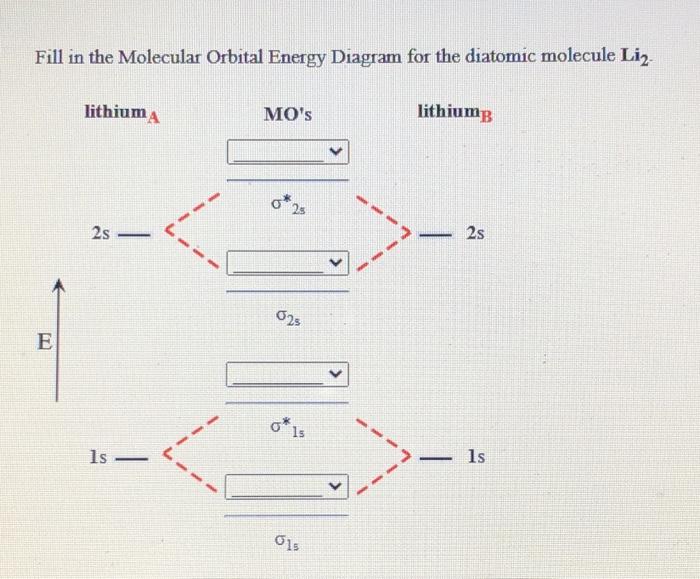
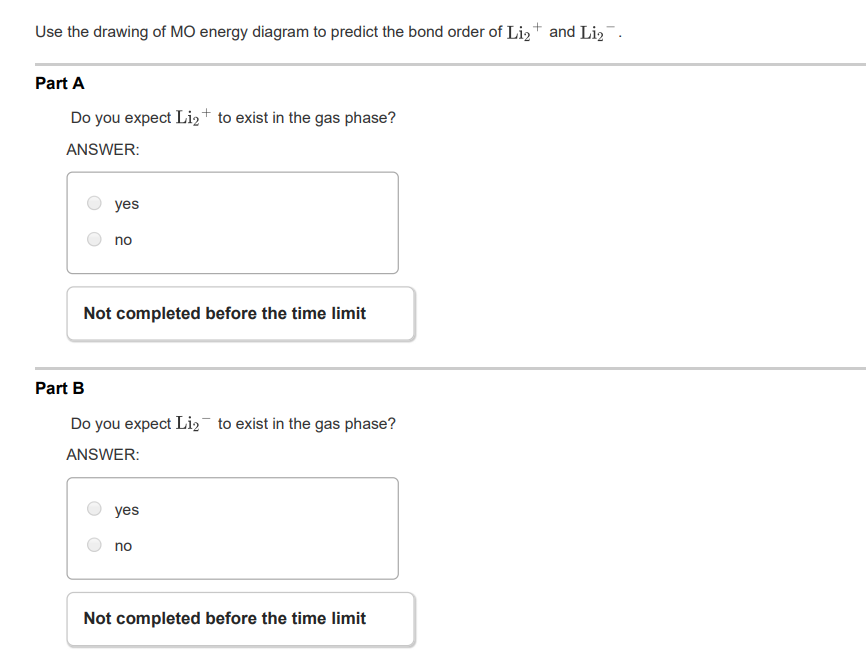
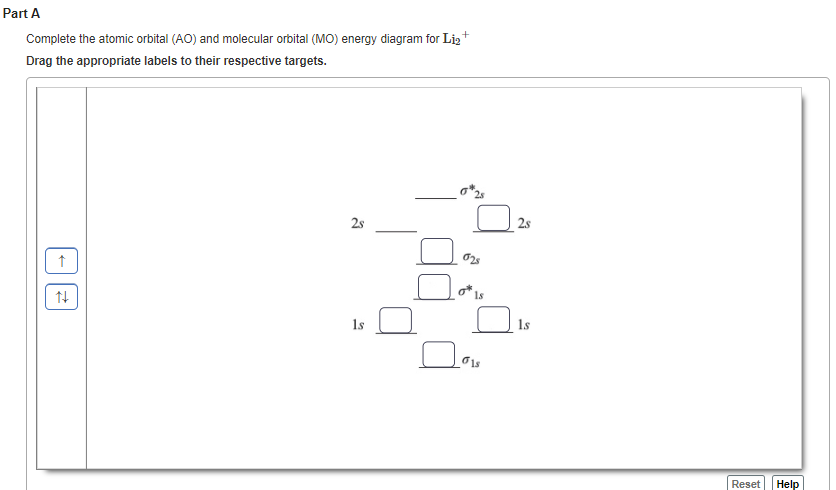
37 complete the atomic orbital (ao) and molecular orbital (mo) energy diagram for li2+.
Figure 2.2. 6: Molecular orbital energy diagram for the H 2 molecule. The energy of an electron in one of the atomic orbitals is α, the Coulomb integral. (2.2.6) α = ∫ φ 1 H φ 1 d τ. where H is the Hamiltonian operator. Essentially, α represents the ionization energy of an electron in atomic orbital φ 1 or φ 2. We give 100% refund for an assignment that we can’t complete that had been paid for. Will my paper be plagiarized? We do not take the issue of plagiarism rightly. As a company we try as much as possible to ensure all orders are plagiarism free. All our papers are written from scratch thus producing 100% original work. We also have a plagiarism detection system where all our papers are ...
Atkins Physical Chemistry 10th Solutions - Free ebook download as PDF File (.pdf), Text File (.txt) or read book online for free. physical chemistry

Complete the atomic orbital (ao) and molecular orbital (mo) energy diagram for li2+.
Figure 5.3.1 Molecular Orbitals for the H 2 Molecule (a) This diagram shows the formation of a bonding σ 1 s molecular orbital for H 2 as the sum of the wave functions (Ψ) of two H 1s atomic orbitals. (b) This plot of the square of the wave function (Ψ 2) for the bonding σ 1 s molecular orbital illustrates the increased electron probability density between the two hydrogen nuclei. FREE Answer to 1)Complete the atomic orbital (AO) and molecular orbital (MO) energy diagram for Li2+ and Li2-1 answer · Top answer: Molecular orbital theory is also used to explain bonding in molecules using linear combination of atomic orbitals. According to molecular orbital theory, ... Vollhardt Organic Chemistry Structure Function 6th txtbk.PDF. Download. Vollhardt Organic Chemistry Structure Function 6th txtbk.PDF
Complete the atomic orbital (ao) and molecular orbital (mo) energy diagram for li2+.. Academia.edu is a platform for academics to share research papers. 34 Be2 + Molecular Orbital Diagram.A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbital s method in particular. C would this ion exist. The first ten molecular orbital s may be arranged in order of energy as follow: σ(1s ) ∗(1s ... 18.08.2021 · Burnout starts with a lack of energy, then gradually building into a sense of exhaustion. Burnout, though, is not a recent phenomenon. In 2018, a Gallup study of nearly 7,500 full-time employees found that 23% of them reported feeling burned out at work very often or always, while an additional 44% reported feeling burned out sometimes. Celebrities like Lady Gaga and Beyoncé have spoken out ... Complete Solutions Manual General Chemistry Ninth Edition ... Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION Ebbing/Gammon. Uploaded by. Sofia Uribe Sanchez. connect to download. Get pdf ..... Preface This Complete Solutions Manual provides worked-out answers to all of the problems that appear in General Chemistry, 9th Edition, by Darrell D. Ebbing and ... DOWNLOAD …
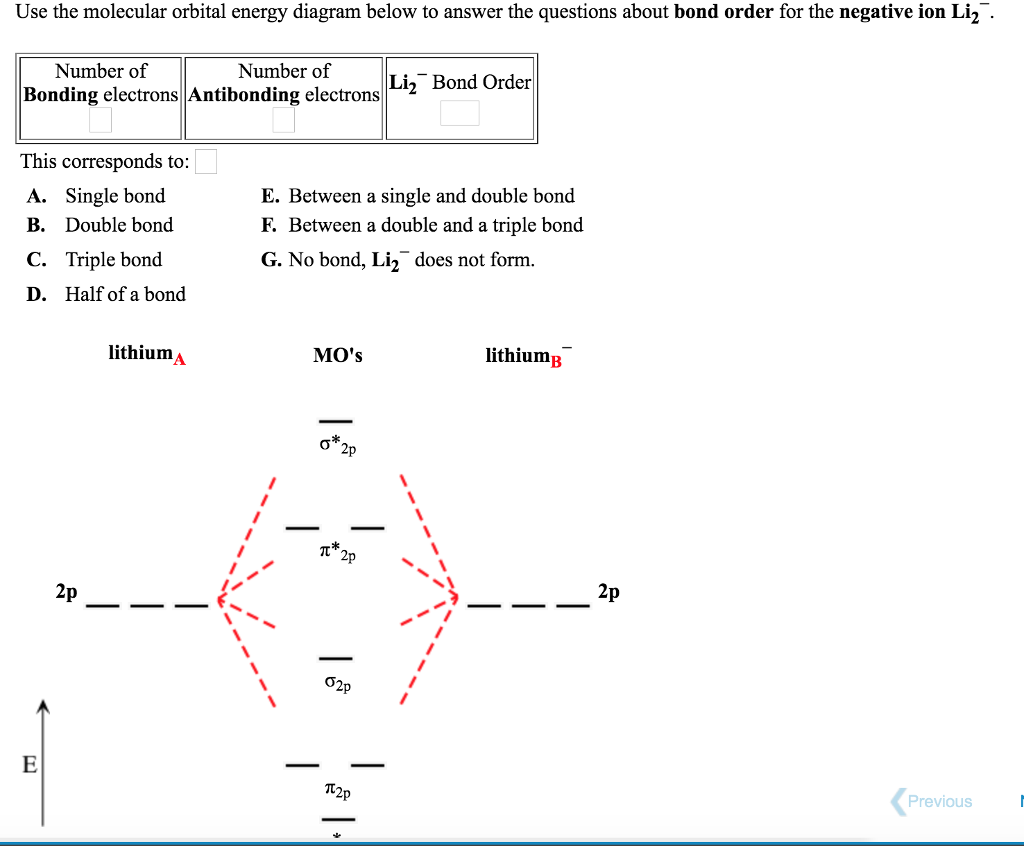
11 Dec 2019 — Get the detailed answer: complete the atomic orbital (ao) and molecular orbital (mo) energy diagram for li2+.1 answer · Top answer: The Molecular Orbital Theory states that each atom tends to combine together to form molecular orbitals. In MOT, electrons in a molecule are not assigned ... Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu. When two orbitals are added, the result is stable bonding molecular orbital and when orbitals are subtracted, it is called unstable anti-molecular bonding (*) which has more energy than the latter one. Considering the energy level diagram, the configuration of N2 is σ1S2, σ *1S2, σ2S2, σ*2S2, π2Px2, π2Py2, σ2Pz1. Conclusion Question: 1)Complete the atomic orbital (AO) and molecular orbital (MO) energy diagram for Li2+ and Li2-. This problem has been solved! See the answer ...
Molecular orbital diagram for ne2 2 . Molecular orbital diagram for ne2 2 Molecular orbital diagram for ne2 2 ... One mole equals the atomic mass or molecular mass in grams. (c) Hg, 200.59 g (d) H2O, 18.02 g (a) Ti, 47.88 g (b) Br2, 159.81 g 3.45 (a) 1.00 g Cr x 3.46 There are 2 ions per each formula unit of NaCl. (2.5 mol)(2 mol ions/mol) = 5.0 mol ions 3.47 There are 2 K+ ions per each formula unit of K2SO4. 2 mol K + 1.45 mol K 2 SO4 x = 2.90 mol K + 1 mol K 2 SO4 3.48 There are 3 ions (one Mg2+ and 2 ... 1 answerProblem: Draw the molecular orbital (MO) energy diagram for Li2+. FREE Expert Solution. The total number of valence electrons present in Li2+ is:. 17.11.2021 · Molecular orbital diagram practice worksheet
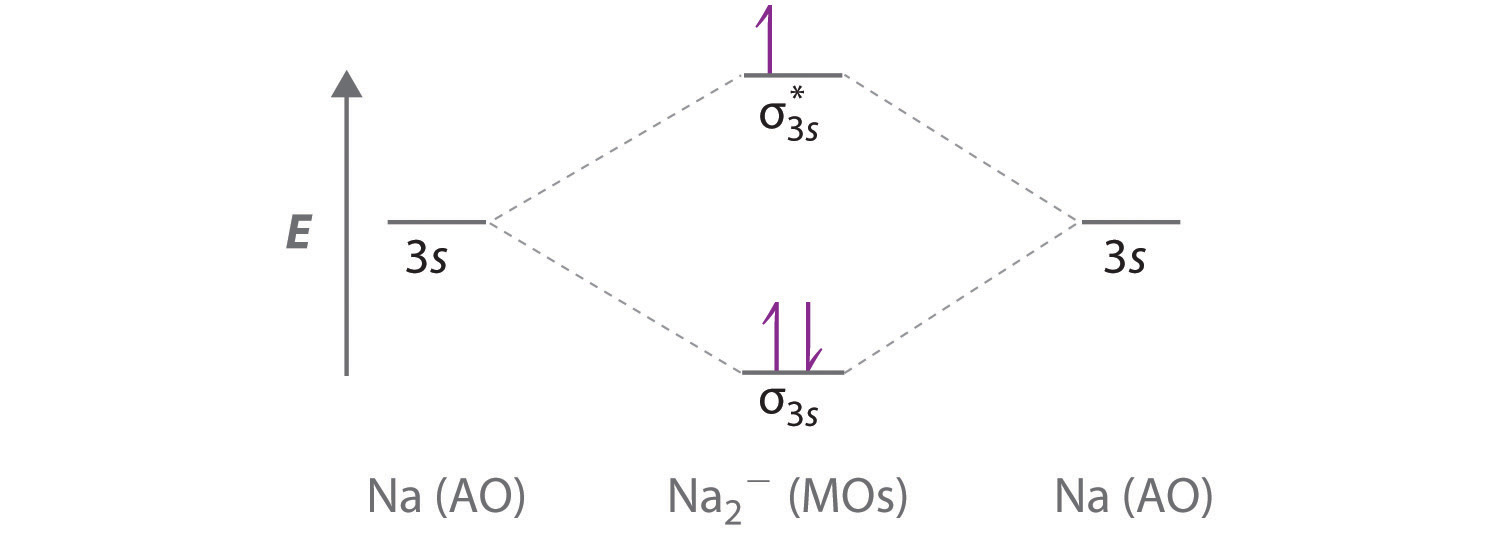
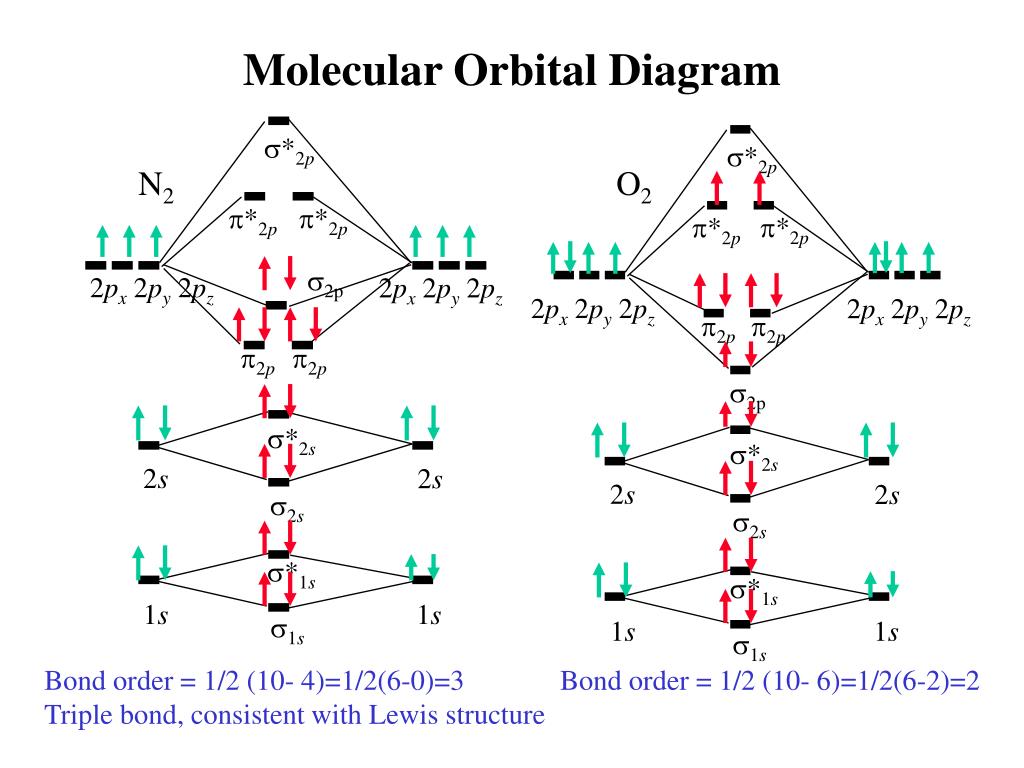
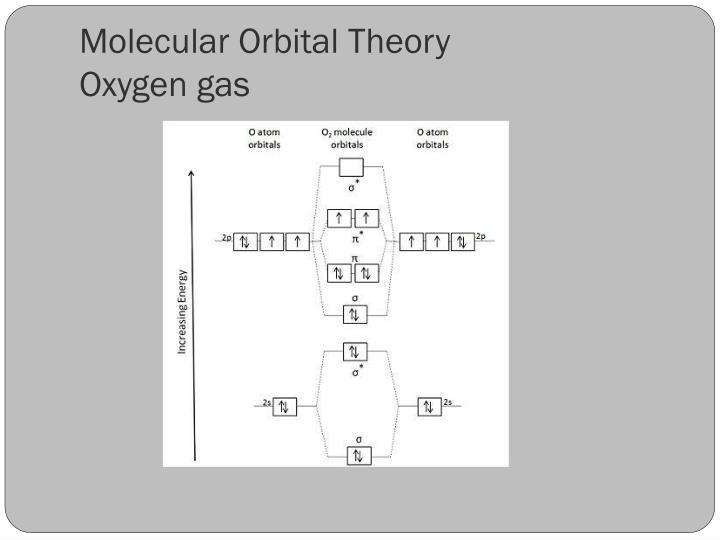
Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9. It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2 ...
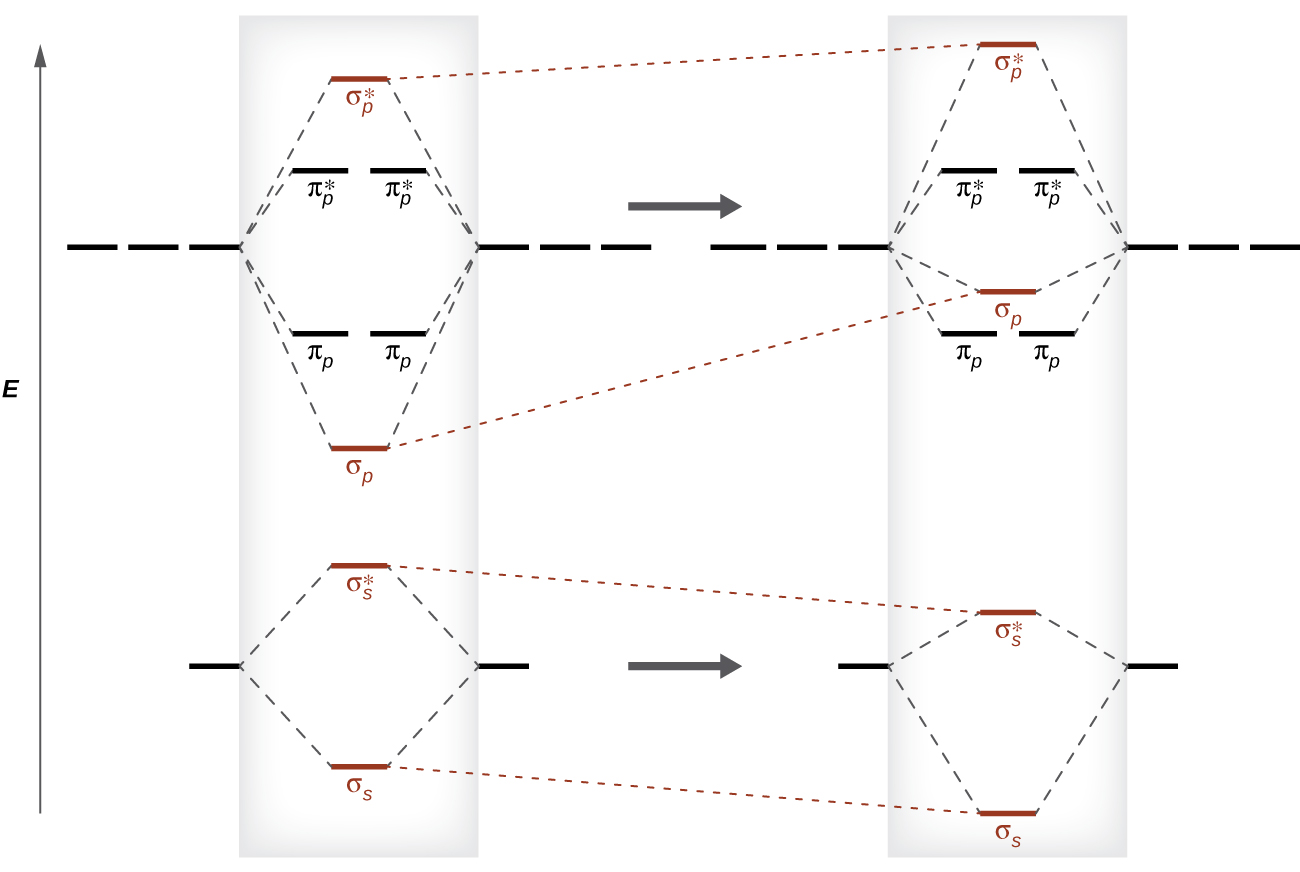
19 Aug 2019 — In general the energy difference between a bonding and anti bonding orbital pair becomes larger as the overlap of the atomic orbitals increase.
Our analysis, using the latest available low-redshfit data and local constraints from atomic clock and weak equivalence principle experiments, shows that the two possible deviations of the dark energy equation of state are constrained to be $\log_{10}{(1+w_0)_V}<-7.85$ and $\log_{10}{(1+w_0)_C}<-0.85$, respectively for the rolling tachyon and ...
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
Well, build the molecular orbital (MO) diagram.Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbital s overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbital s. Draw a molecular orbital diagram of ${N_2}$ or ${O ...

The One That Is Lower In Energy Is Called The Bonding Orbital The One Higher In Energy Is Called An Antibonding Orbital These Two Ppt Download
Atomic number = Number of protons. For example, in a sodium atom, there are 11 electrons and 11 protons. Thus the atomic number of Na atom = number of electrons = number of protons = 11. Atomic Number Orbital Energy Levels. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others.
Vollhardt Organic Chemistry Structure Function 6th txtbk.PDF. Download. Vollhardt Organic Chemistry Structure Function 6th txtbk.PDF
FREE Answer to 1)Complete the atomic orbital (AO) and molecular orbital (MO) energy diagram for Li2+ and Li2-1 answer · Top answer: Molecular orbital theory is also used to explain bonding in molecules using linear combination of atomic orbitals. According to molecular orbital theory, ...
Figure 5.3.1 Molecular Orbitals for the H 2 Molecule (a) This diagram shows the formation of a bonding σ 1 s molecular orbital for H 2 as the sum of the wave functions (Ψ) of two H 1s atomic orbitals. (b) This plot of the square of the wave function (Ψ 2) for the bonding σ 1 s molecular orbital illustrates the increased electron probability density between the two hydrogen nuclei.

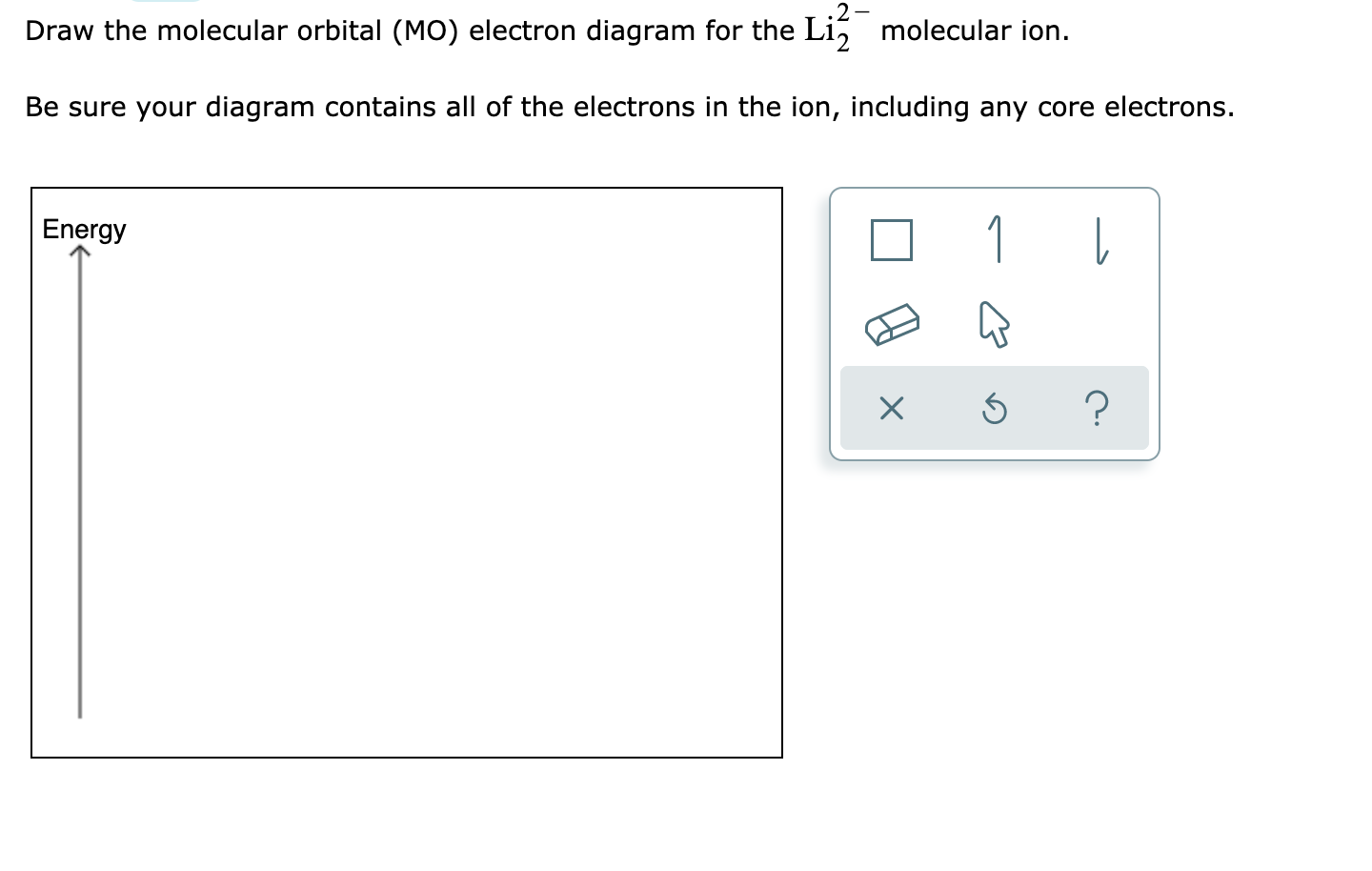
1 Complete The Atomic Orbital Ao And Molecular Orbital Mo Energy Diagram For Li2 And Li2 Homeworklib

Solved Chapter 10 Problem 42e Solution Masteringchemistry Standalone Access Card For Principles Of Chemistry 2nd Edition Chegg Com

Complete The Atomic Orbital Diagram For The Ground State Electronic Configuration Of Chlorine Answer Bank Energy Homeworklib












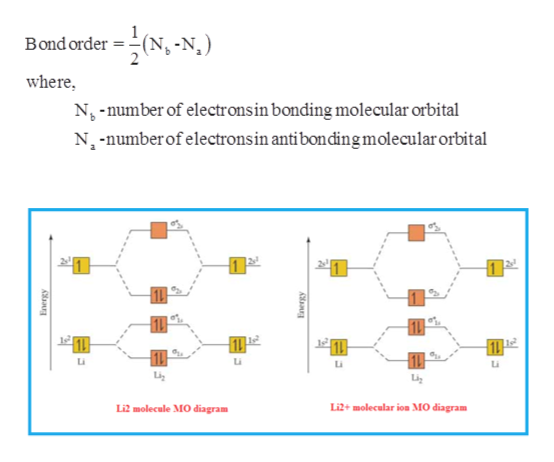




0 Response to "37 complete the atomic orbital (ao) and molecular orbital (mo) energy diagram for li2+."
Post a Comment