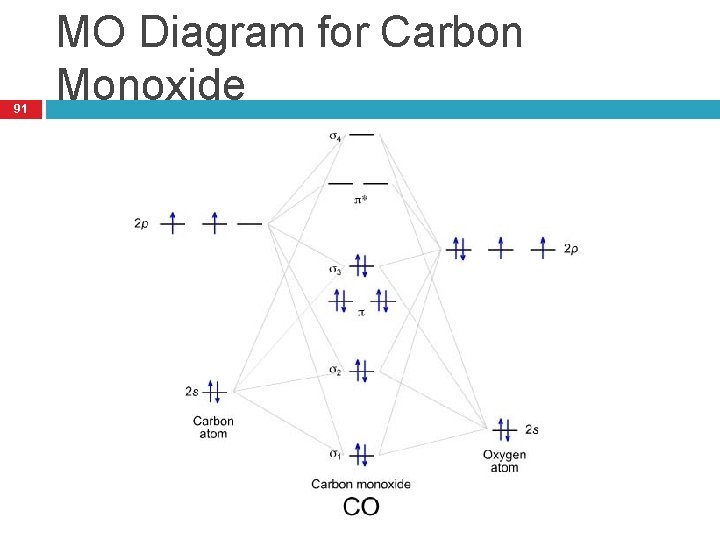
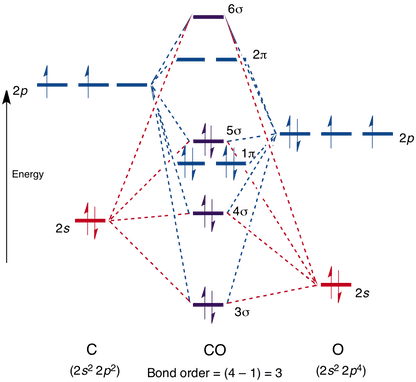
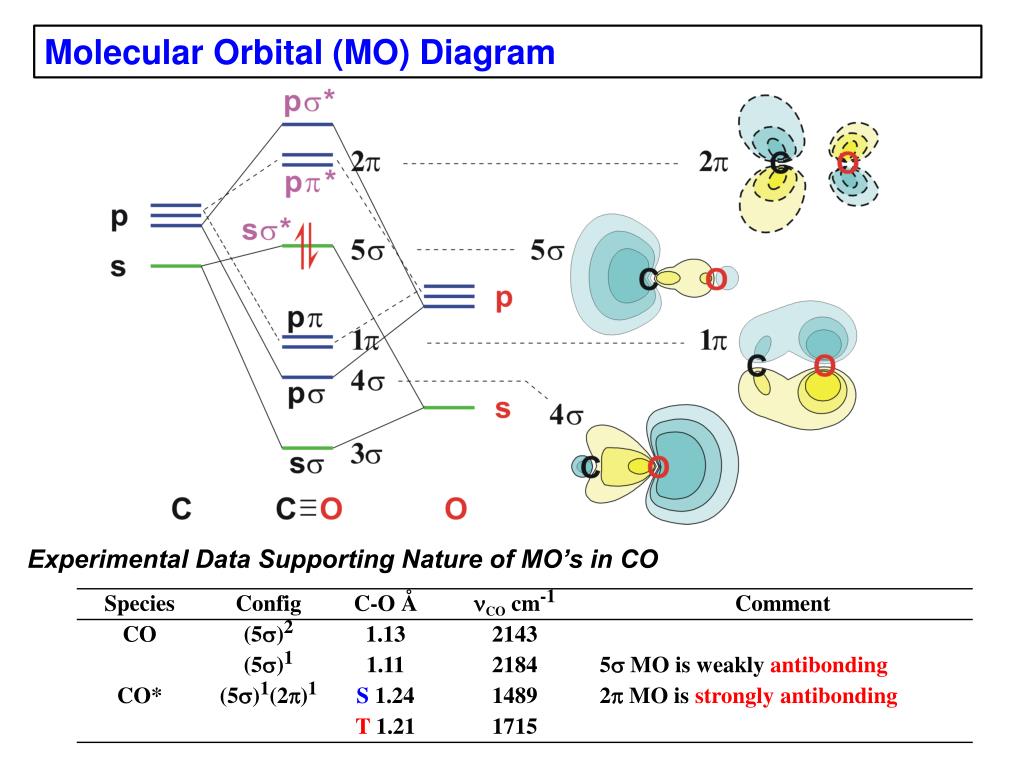
38 co molecular orbital diagram
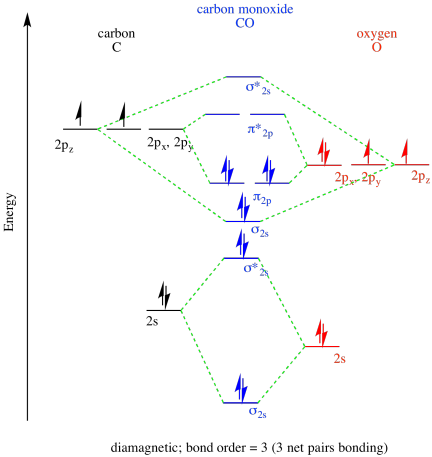
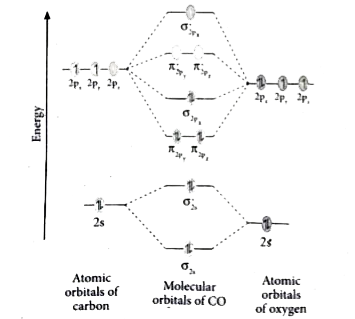
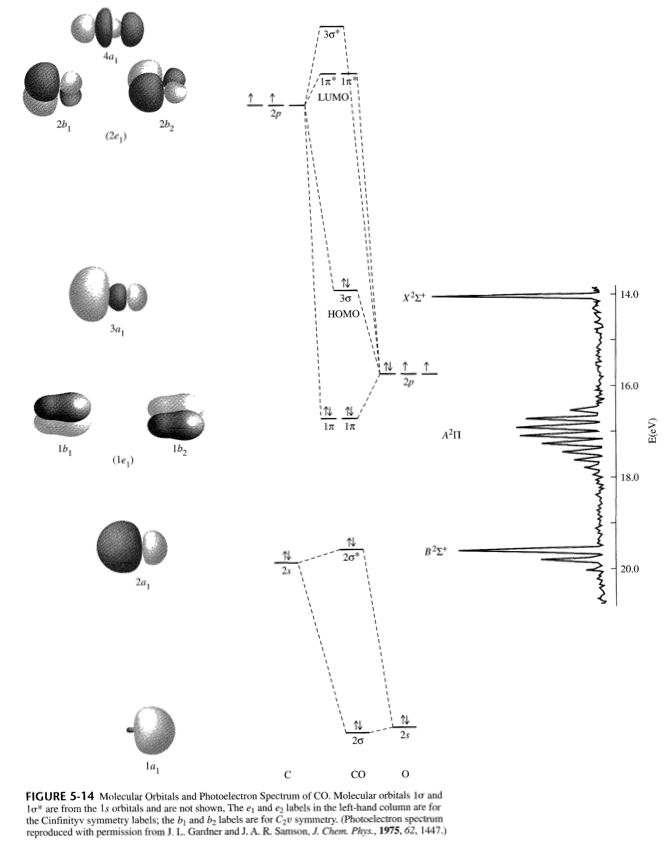
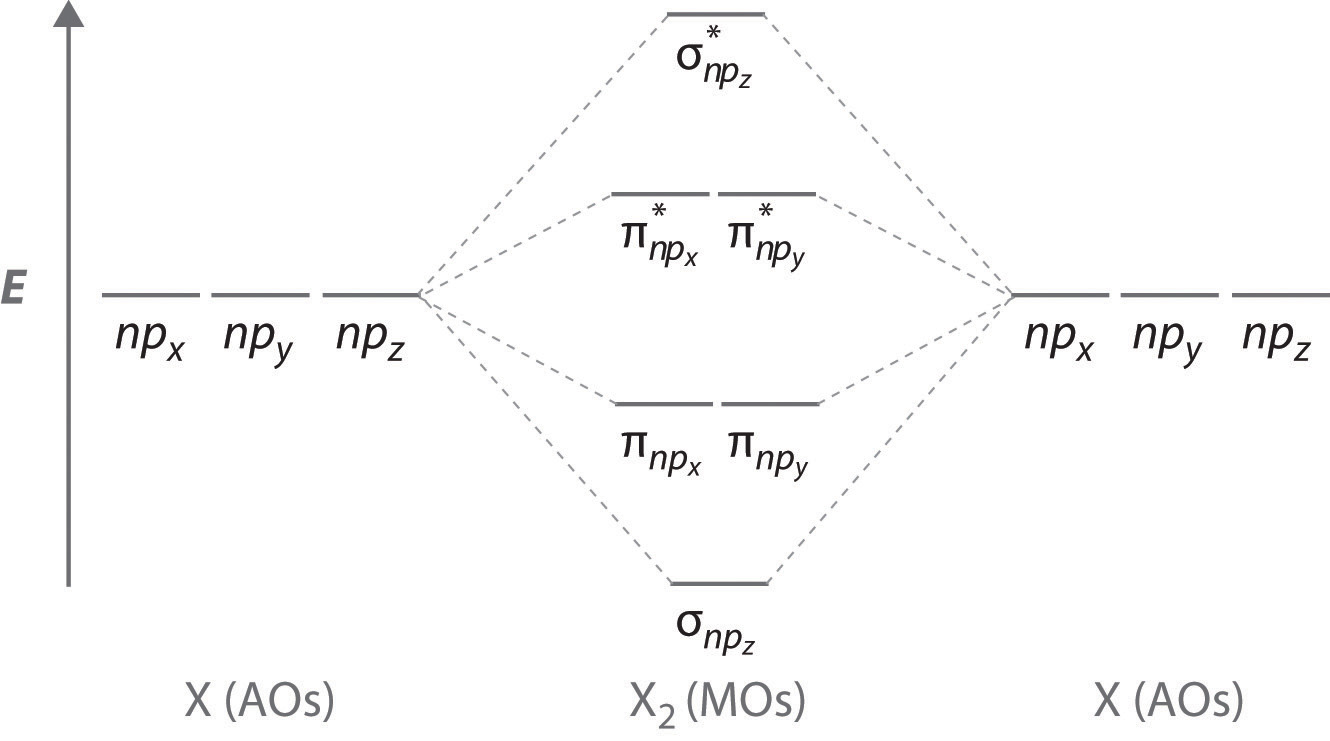
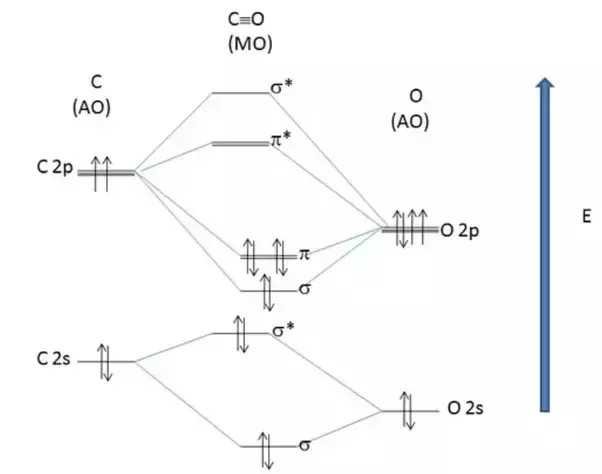
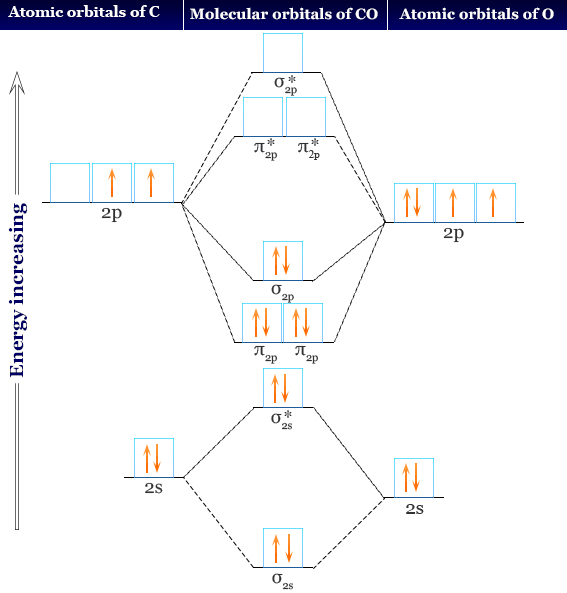
In the case of CO, the 2s atomic orbital on Oxygen is much lower in energy than the 2s atomic orbital in carbon. The discrepancy in energies allows the π2px & π2py bonding molecular orbitals to sink lower in energy than the “ σ*2s MO” in the MO diagram of CO. Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
Molecular orbital diagram of co. Tricky chemistry basics. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular. The course introduces the three key spectroscopic ...
Co molecular orbital diagram
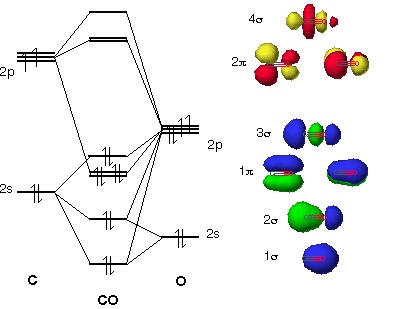
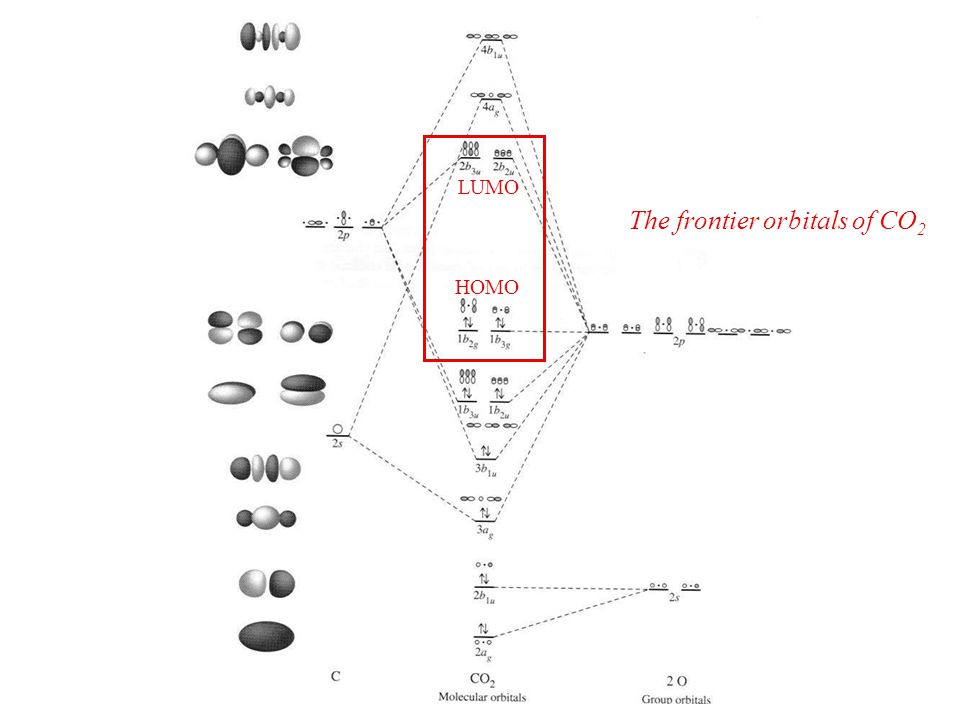
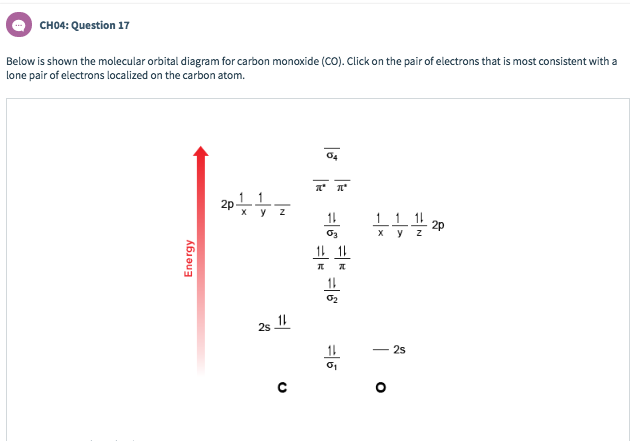
The molecular orbital diagram of carbon monoxide, CO, is show below. Which overlap is strongest? During the axial overlap of p-p orbitals, the electron density increases around the axis, so the bond formed is the strongest. Therefore, the strongest bond formed is when p-p orbital overlap occurs. Final answer: The correct answer is Option B- 2p ... Orbital-orbital Interactions and Symmetry Adapted Linear Combinations; ... Molecular orbitals in Carbon Monoxide. CONTROLS > Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. Point out key differences between the diagrams and use the diagram to explain why $\ce{CO}$ acts as a two-electron donor through carbon rather than through oxygen. Understandably, the key difference between these molecules is that $\ce{CO}$ is heteronuclear, and thus will have differences in energy between the molecular orbital and the atoms.
Co molecular orbital diagram. 12-12 This video describes the molecular orbital theory diagram of CO, placing emphasis on how MO theory differs for homo and heteronuclear diatomics A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not. . combinations such as CO and NO show that the 3σg MO is higher in energy. Mulliken came up with theory known as Molecular Orbital Theory to explain questions like above. Molecular orbital diagram of CO and charge localisation. Ask Question Asked 1 year, 5 months ago. Active 1 year, 5 months ago. Viewed 110 times ... My question concerns the interpretation of the Molecular Orbital of CO. I think I find it clear how you build it but I have some concerns about how you rationalize it. Molecular Orbital Diagram of CO. TAGS; Molecular Orbital Diagram; Previous article Wohl-Ziegler Bromination. Next article Molecular Orbital Diagram of NO. All About Chemistry. https://allaboutchemistry.net. Hello Reader! Thanking for reading this post, If you find it to be informative, pls share it and visit our website.
Point out key differences between the diagrams and use the diagram to explain why $\ce{CO}$ acts as a two-electron donor through carbon rather than through oxygen. Understandably, the key difference between these molecules is that $\ce{CO}$ is heteronuclear, and thus will have differences in energy between the molecular orbital and the atoms. Orbital-orbital Interactions and Symmetry Adapted Linear Combinations; ... Molecular orbitals in Carbon Monoxide. CONTROLS > Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. The molecular orbital diagram of carbon monoxide, CO, is show below. Which overlap is strongest? During the axial overlap of p-p orbitals, the electron density increases around the axis, so the bond formed is the strongest. Therefore, the strongest bond formed is when p-p orbital overlap occurs. Final answer: The correct answer is Option B- 2p ...

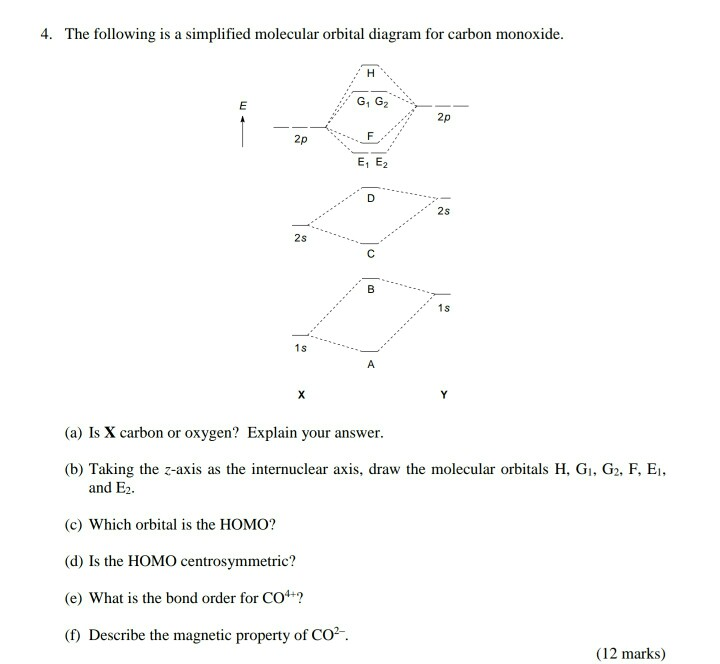
Draw The Molecular Orbitals For Co In Order Of Energy And Fill Them With The Appropriate Number Of Electrons Label The Orbitals The Best You Can As Sigma Or Pi And As

Solved Question 43 Molecular Orbitals 12 4 4 4 24 Points Draw The Molecular Orbital Diagram For Carbon Monoxide Co Draw Your Diagram With Carbon On The Left Oxygen On The Right And Coin The Center Mote

Draw The Molecular Orbital Diagram For Indicate The Point Group Symmetry And Draw With Bonding And Homeworklib
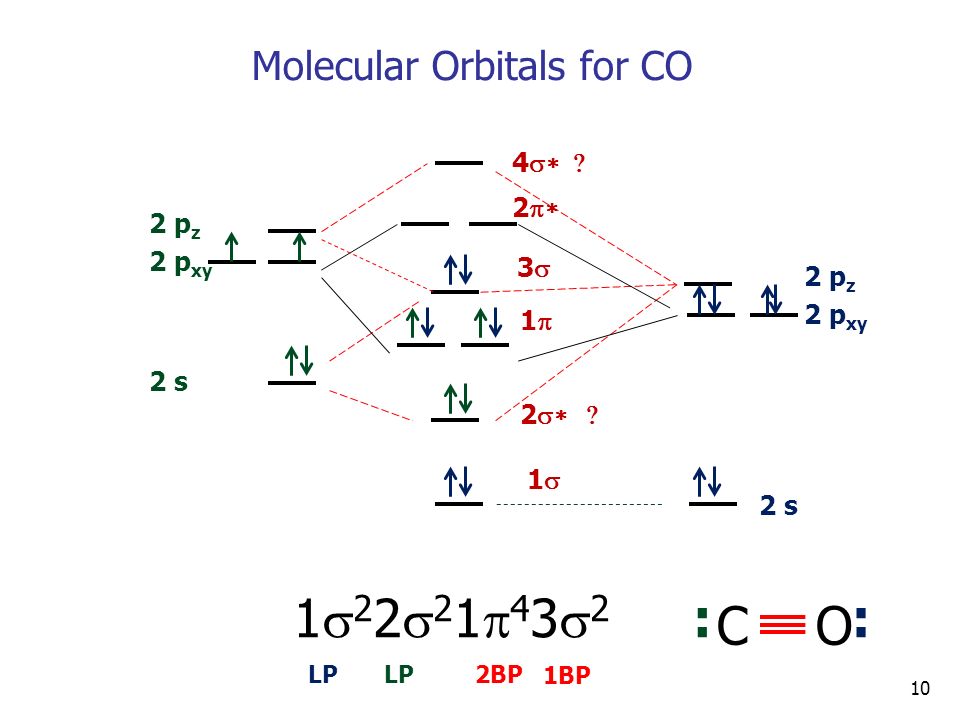
Molecular Orbitals Of Heteronuclear Diatomics The Molecular Orbitals Of Heteronuclear Diatomics Hf Co Cn Etc Can Be Predicted Using The Same Principles Ppt Download








0 Response to "38 co molecular orbital diagram"
Post a Comment