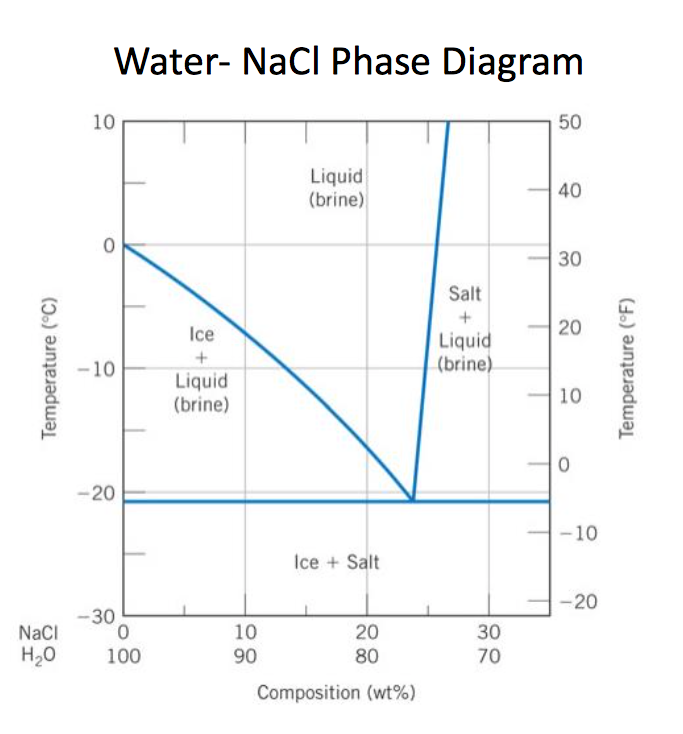
39 salt water phase diagram
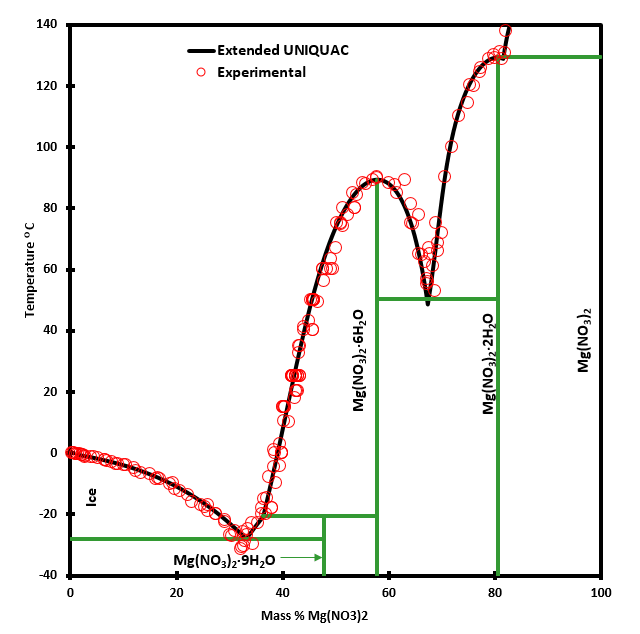
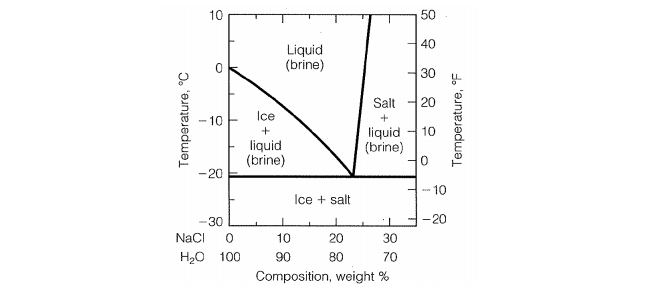
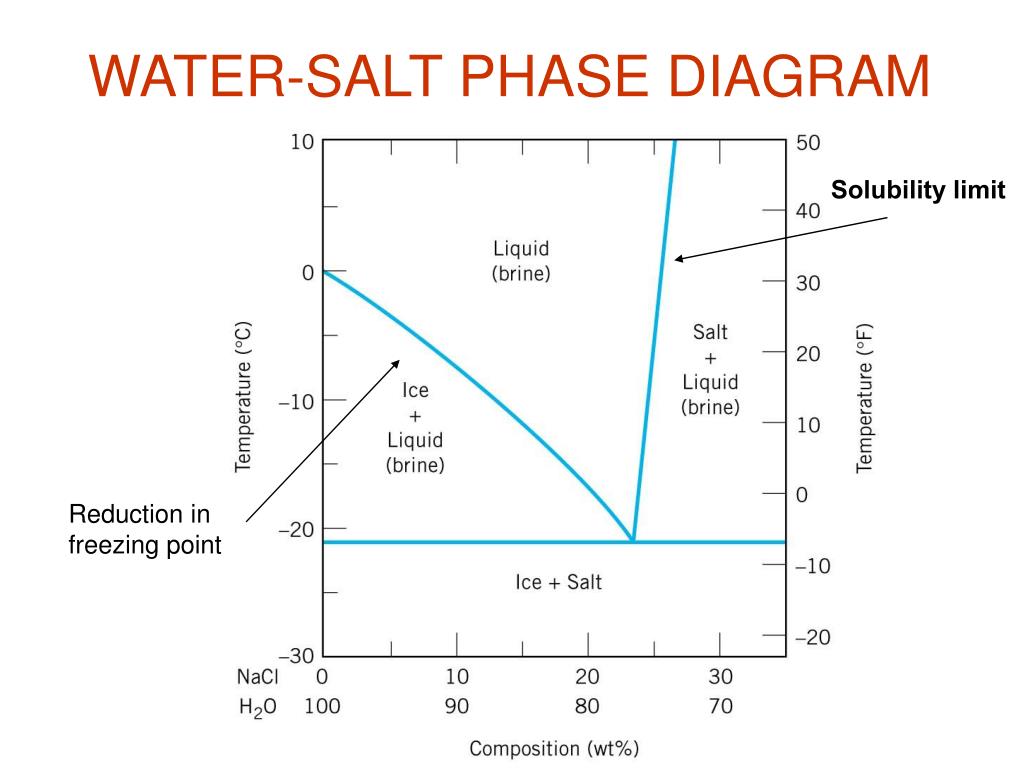
Phase Diagram of Salt Water · There is an eutectic composition around 27 wt % NaCl or salt dissolved in the water. · At the eutectic point, the melting or ... A typical ternary phase diagram (glycerol/NaCl/water) is shown in Fig. 1. The curve of freezing point vs wt% solute is referred to as an isopleth. In ternary phase diagrams the position and shape of isopleths depends on the wt% ratio ( R) of CPA to salt that is used. Fig. 1 depicts isopleths for R = 0, 1, and 15.
FTsalt - FACT Salt Phase Diagrams (351) Click on a system to display the phase diagram.

Salt water phase diagram
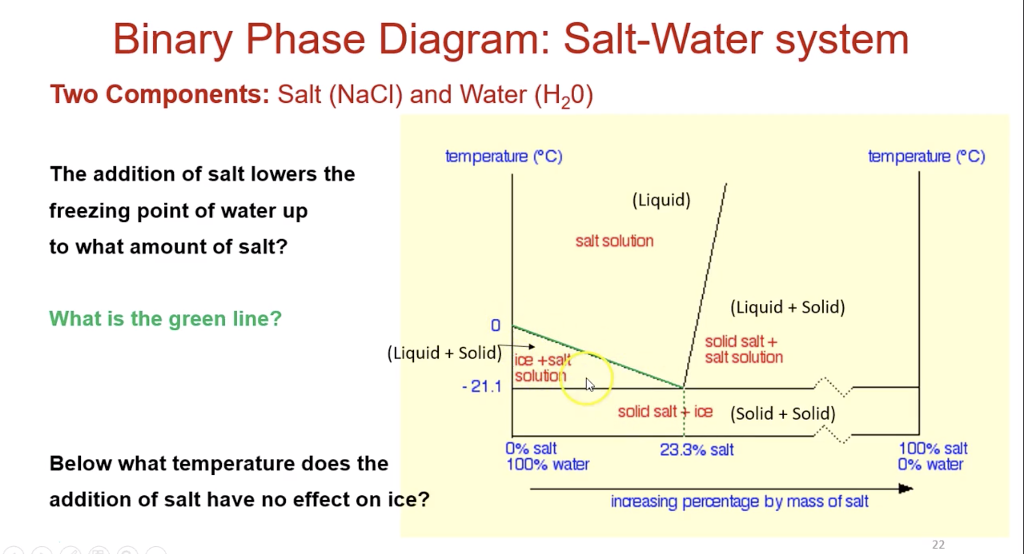
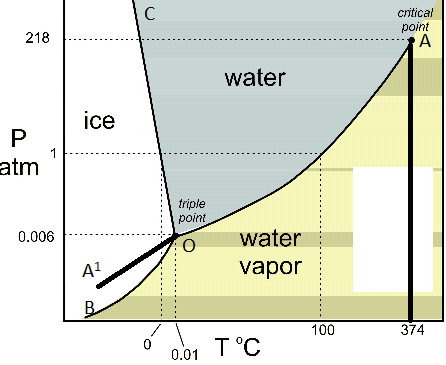
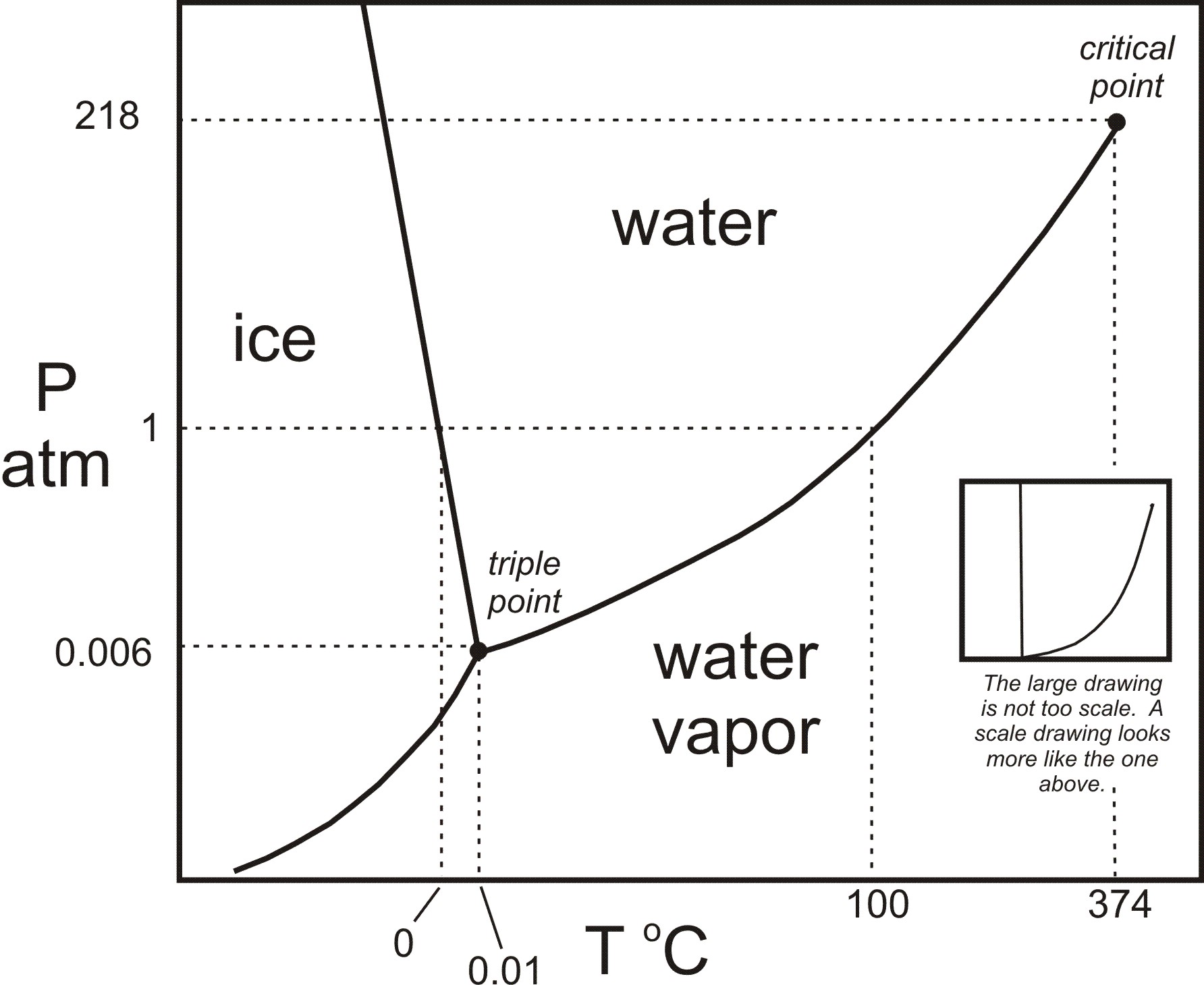
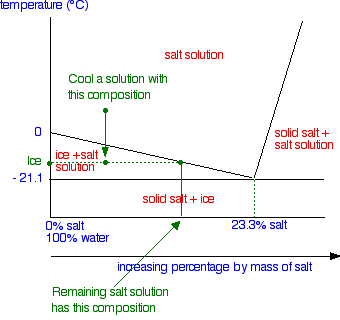
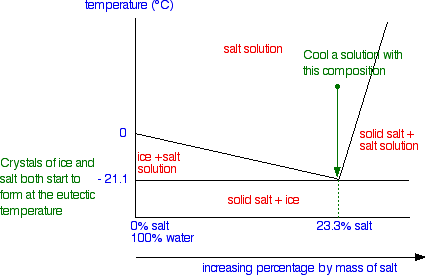
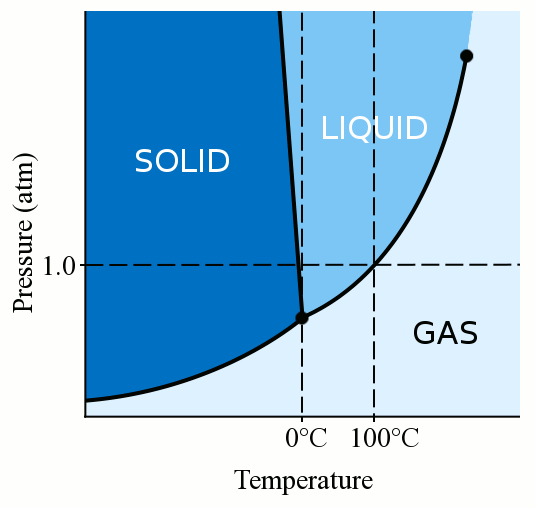
In the sodium chloride – water system, one eutectic point and one peritectic point is found. These points are shown in the phase diagram to the right. Phase diagram of water Note: for H2O melting point decreases with increasing pressure, for CO2 melting point increases with increasing pressure. WATER Covers ~ 70% of the earth’s surface Life on earth depends on water Water is a “universal” solvent Easily polluted; hard to purify. Most phase diagrams at 1 atm Reading: West 11-12 Chem 253, UC, Berkeley Phases Homogeneous portion of the system with uniform physical and chemical characteristics Salt – water Salt NaCl A difference in either physical or chemical properties constitutes a phase Water and ice FCC and BCC polymorphic forms of an element
Salt water phase diagram. Cooling salt solution more concentrated than the eutectic composition — The phase diagram is interpreted in just the same way - except that this time, salt ...The phase diagram for sodium... · What the lines mean · Using Phase Diagrams The phase diagram for water is shown in the Figure below . Figure 13.26. Phase diagram for water. Notice one key difference between the general phase diagram and the phase diagram for water. In water’s diagram, the slope of the line between the solid and liquid states is negative rather than positive. The reason is that water is an unusual ... ... desalination processes are based on the fact that ice crystals are made up of essentially pure water when the temperature of saline water is lowered to its ... The labelled areas in the phase diagram. These areas all show what you would see if you had a particular mixture of salt and water at a given temperature. For example, if the temperature was below -21.1°C, you would always see a mixture of solid salt and ice. There would never be any liquid whatever proportions of salt and water you had.
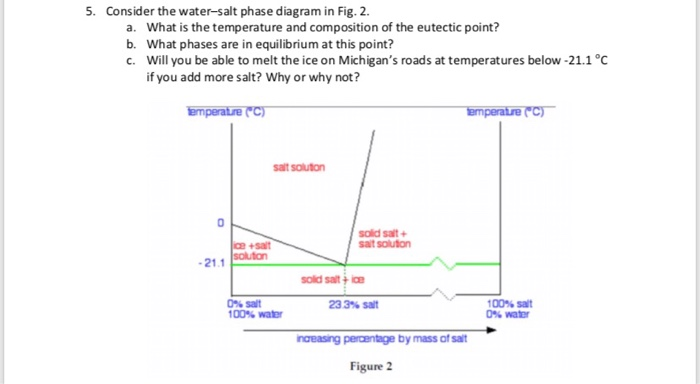
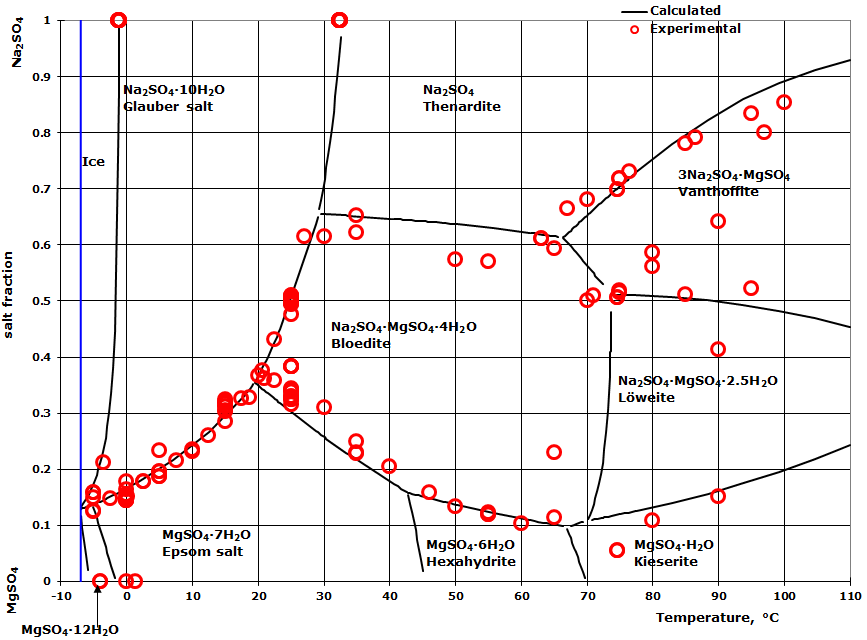
Transcribed image text: 2. This question pertains to the salt-water phase diagram shown below: Liquid (brine) Salt Ice Temperature (°C) Liquid (brine) Temperature (°F) Liquid (brine) Ice + Salt 20 NECE-300 1 0 HO 10090 20 30 Composition (wt%) (a) 100 g of salt is added to a beaker containing 750 g of water at 5°C and sufficiently mixed to dissolve all of the salt. COMPUTE WATER PHASE SALT CONTENT Water Phase Salt can be calculated by using either percent or grams of salt and moisture from the analysis. 100 % % % X Salt Moisture Salt WPS + = or X100 gSalt gMoisture gSalt WPS + = Example: Using the example above where a10 gram sample of smoked fish was found to have 60% moisture and 2.88% salt (0.288 g ... The phase diagram for the system at 25°C is shown below. The abscissa in the diagram is the Na 2 O fraction. The ordinate is the number of moles of water per mole of salt. The dashed blue lines are tie-lines connecting the saturated liquid with the corresponding solid. Only the tie-lines marking the transition to another stable solid phase are ... This problem has been solved! See the answer. 1) According to the water-salt phase diagram, what is the approximate solid-solubility of water in NaCl? 2) Excluding the pure components of water and salt, how many stoichiometric compounds are stable in the water-salt system? Show transcribed image text.
Most phase diagrams at 1 atm Reading: West 11-12 Chem 253, UC, Berkeley Phases Homogeneous portion of the system with uniform physical and chemical characteristics Salt – water Salt NaCl A difference in either physical or chemical properties constitutes a phase Water and ice FCC and BCC polymorphic forms of an element Phase diagram of water Note: for H2O melting point decreases with increasing pressure, for CO2 melting point increases with increasing pressure. WATER Covers ~ 70% of the earth’s surface Life on earth depends on water Water is a “universal” solvent Easily polluted; hard to purify. In the sodium chloride – water system, one eutectic point and one peritectic point is found. These points are shown in the phase diagram to the right.
How Do Sublimation Curve And Triple Point Affect The Phase Diagram Of Water Upon Adding Salt To Water Quora

Teaching A New Technology Eutectic Freeze Crystallization By Means Of A Solved Problem Sciencedirect
















0 Response to "39 salt water phase diagram"
Post a Comment