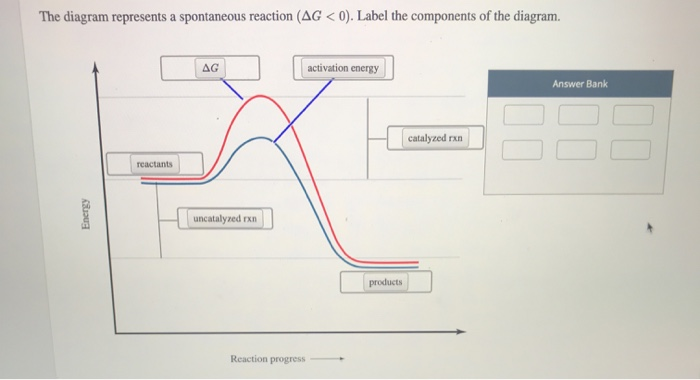
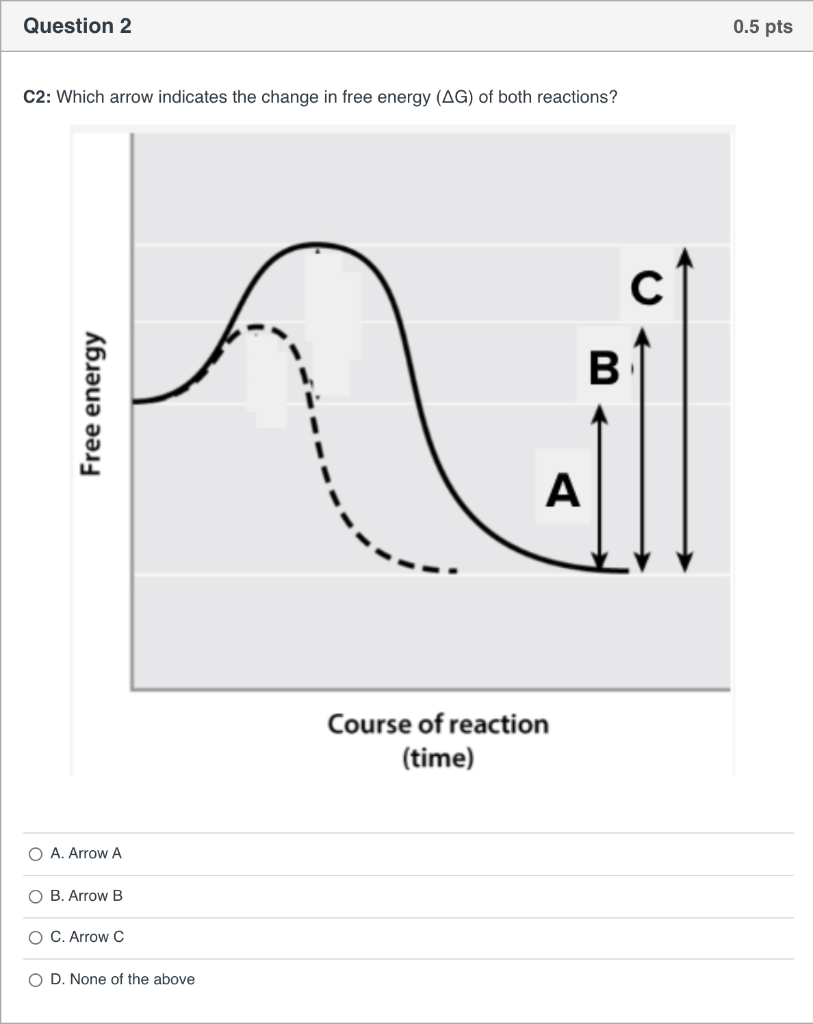
34 the diagram below represents a spontaneous reaction (δg
chem1101 2014 j 12 june 2014 the diagram below represents this reaction involves an increase in the number of moles of gas so Δs will be positive as Δh = Δg tΔs this means that Δh Δg for the other reactions there is a decrease in the number of moles of has so Δs is negative Δh = Δg tΔs this means that Δh Δg
Gibbs free energy and spontaneity. When a process occurs at constant temperature and pressure , we can rearrange the second law of thermodynamics and define a new quantity known as Gibbs free energy: where is enthalpy, is temperature (in kelvin, ), and is the entropy. Gibbs free energy is represented using the symbol and typically has units of .
Exergonic reactions are also called spontaneous reactions, because they can occur without the addition of energy. Reactions with a positive ∆ G (∆ G > 0), on the other hand, require an input of energy and are called endergonic reactions. In this case, the products, or final state, have more free energy than the reactants, or initial state.

The diagram below represents a spontaneous reaction (δg
The potential required to oxidize Cl-ions to Cl 2 is -1.36 volts and the potential needed to reduce Na + ions to sodium metal is -2.71 volts. The battery used to drive this reaction must therefore have a potential of at least 4.07 volts. This example explains why the process is called electrolysis.The suffix -lysis comes from the Greek stem meaning to loosen or split up.
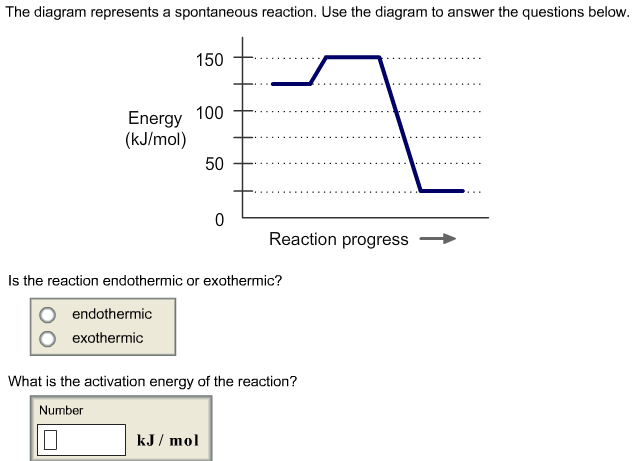
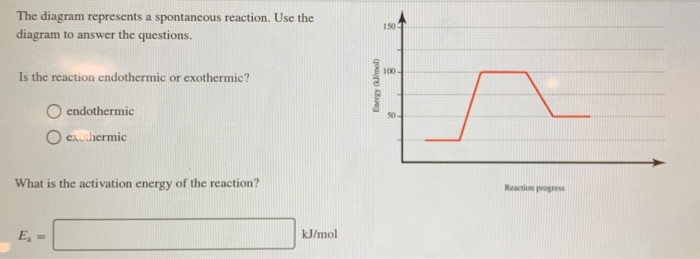
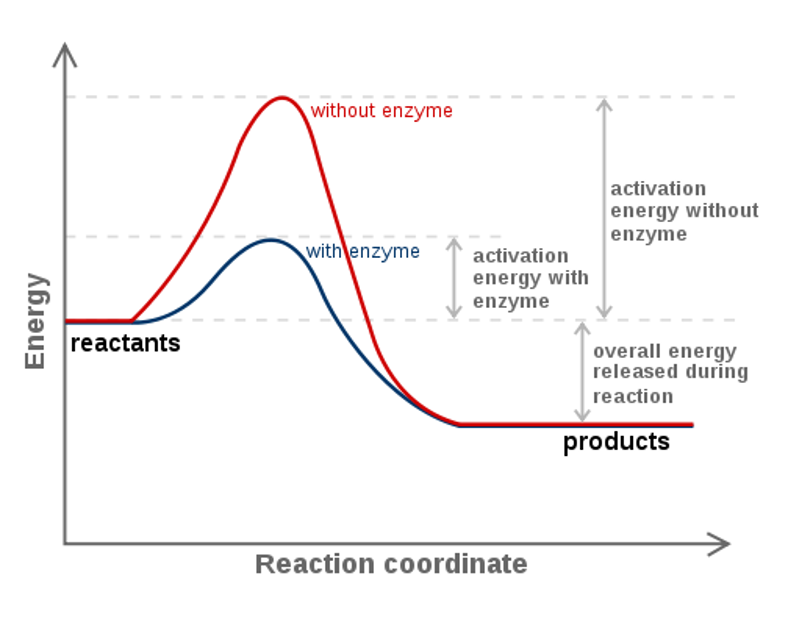
The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. a. Is the reaction endothermic or exothermic? b. What is the activation energy of the reaction?
The diagram below represents a spontaneous reaction δg institution. Chem1101 2014 j 12 june 2014 the diagram below represents this reaction involves an increase in the number of moles of gas so δs will be positive as δh δg tδs this means that δh δg for the other reactions there is a decrease in the number of moles of has so δs is ...
The diagram below represents a spontaneous reaction (δg.
Since ΔG= ΔH-T ΔS: and we have a (-) value of ΔH and a (-) value of ΔS. ΔG = (-) -T(-): Higher values of T will cause the reaction to be less spontaneous, more positive. b. Some reactions that are predicted by their signs of ΔG° to be spontaneous at room temperature do not proceed at a measurable rate at room temperature.
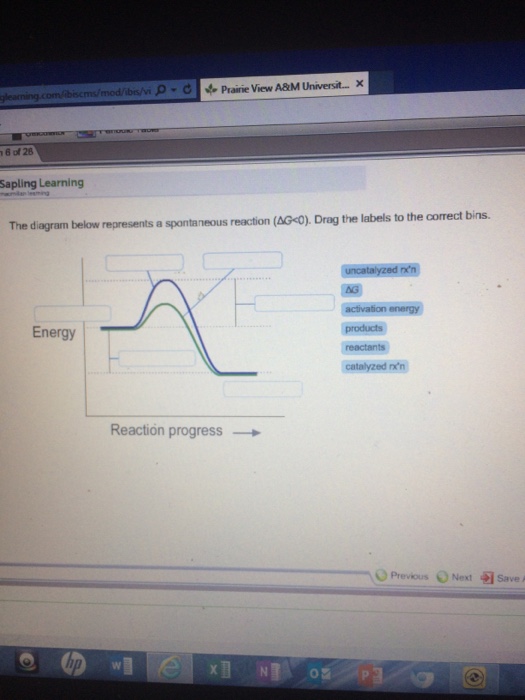
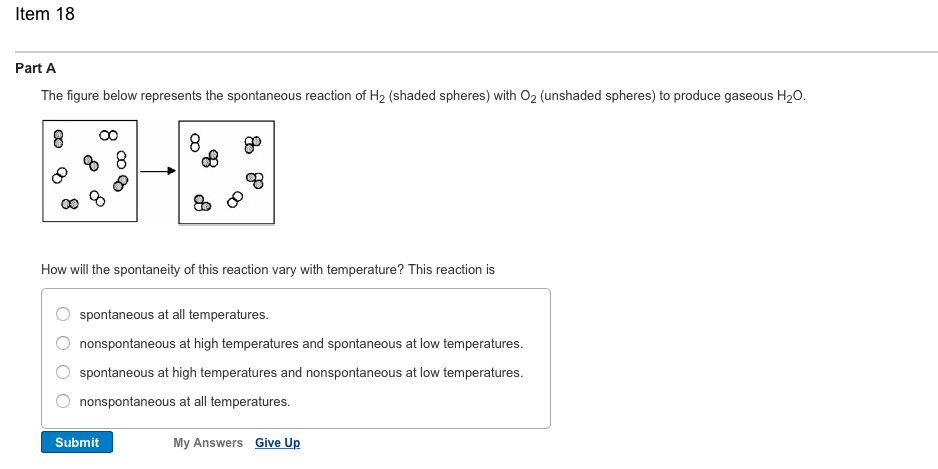
The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free E of activation products reactants catalyzed rx?n. Question: The diagram below represents a spontaneous reaction (deltaG degree < 0).
So, saying a process is spontaneous at "high" or "low" temperatures means the temperature is above or below, respectively, that temperature at which ΔG for the process is zero. As noted earlier, the condition of ΔG = 0 describes a system at equilibrium.
Drag the labels to the correct bins. Chem1101 2014 j 12 june 2014 the diagram listed below represents this reaction involves boost in the number of moles the gas so δs will be confident as δh δg tδs this method that δh δg for the various other reactions there is a diminish in the number of moles of has so δs is an adverse δh δg tδs this method that δh δg.
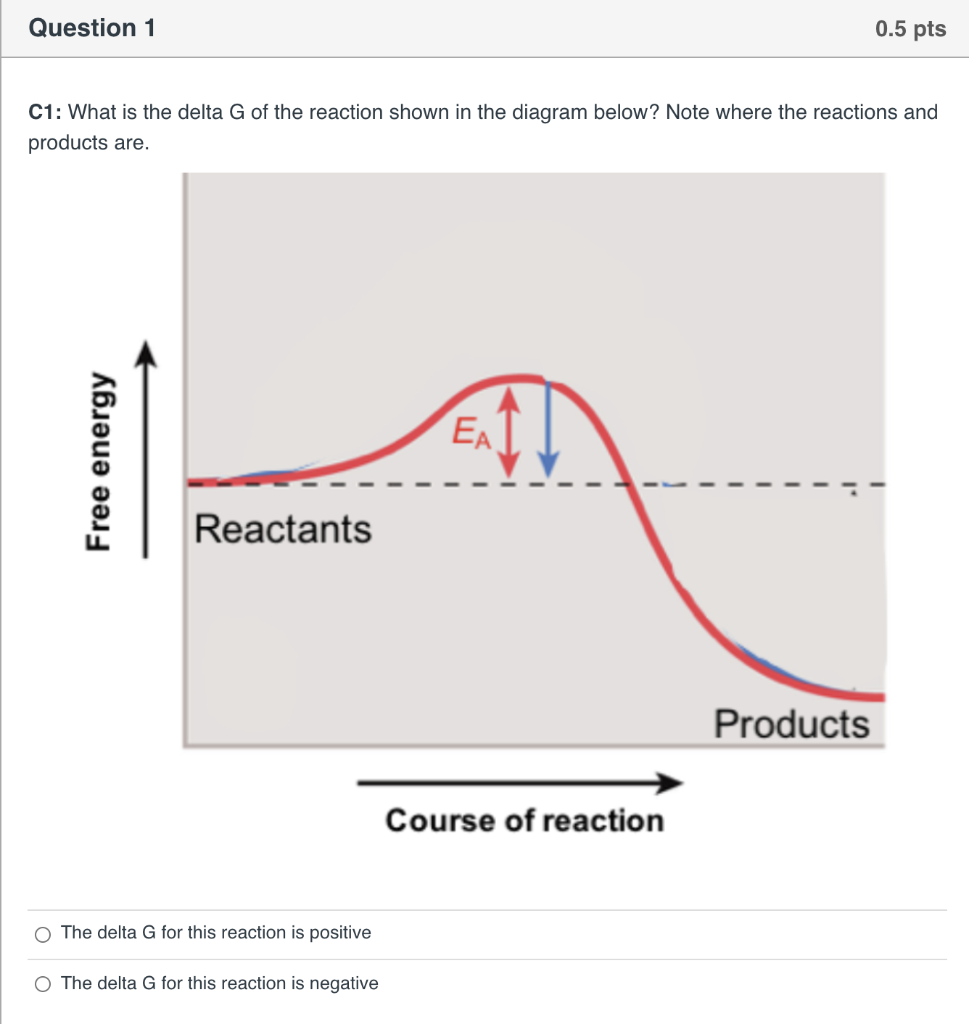
The image represents a spontaneous, gaseous reaction at a constant temperature T KT K. Predict whether ΔHΔH, ΔSΔS, and ΔGΔG for this reaction are positive, negative, or zero. ... A⇌B,ΔG=A⇌B,ΔG= 10.5 kJ/molkJ/mol ... the drive the reaction has under standard conditions to move toward equilibrium from point A to point X in the diagram ...
However for endothermic reactions the reactants are drawn below the products. Solved Label This Diagram Answer Bank Ah E Products Reac The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram or sometimes called a reaction progress curve. Label this diagram energy reaction progress ...
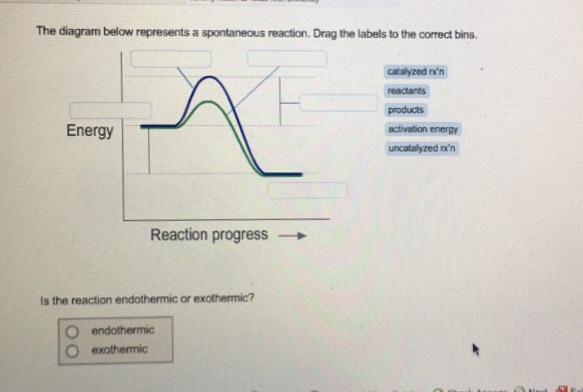
Nov 09, 2021 · 14 Dec 2020 — The diagram below represents a spontaneous reaction. Drag the labels to the correct bins. Is the reaction endo the rmic or exo the rmic? The reaction will only be allowed if the total entropy change of the universe is zero or positive. This is reflected in a negative ΔG, and the reaction is called an exergonic process.
Diagram 2, because it represents a reaction with a high activation energy barrier for molecules to overcome and a very slow reaction rate, even if it is the rmodynamically favorable with ΔG < 0 Diagram 2, because it represents a reaction that is the rmodynamically favorable with ΔH < 0 the products formed are unstable and quickly revert to ...
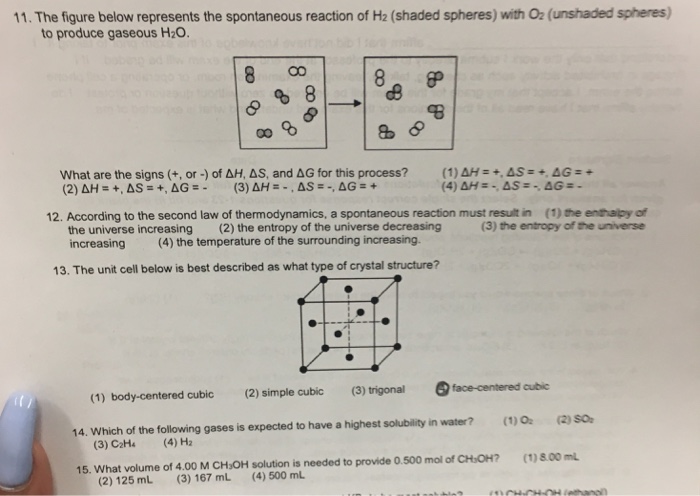
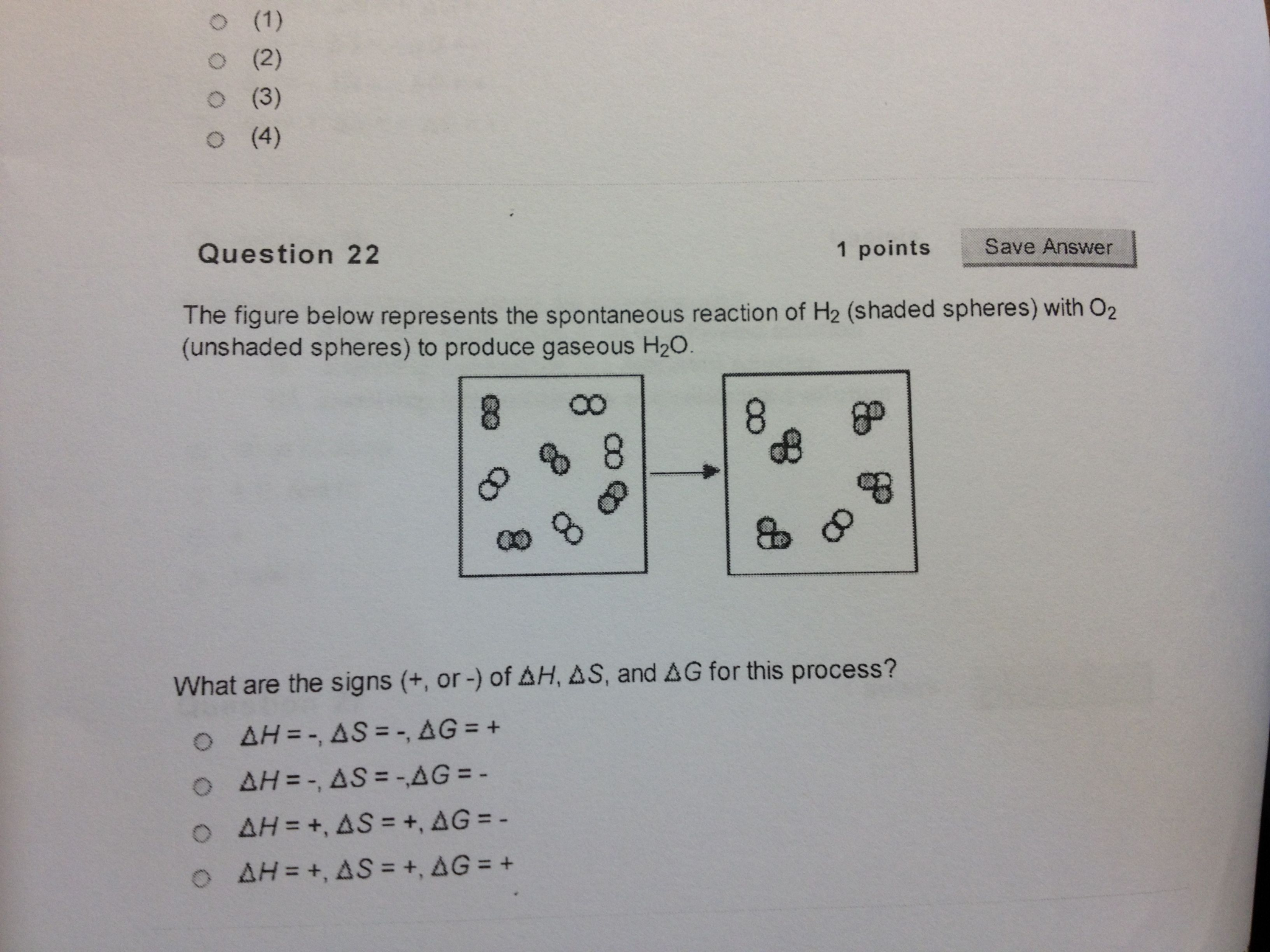
For the reaction below ΔG° = +33.0 kJ,ΔH° = +92.2 kJ,and ΔS° = +198.7 J/K.Estimate the temperature at which this reaction becomes spontaneous. 2 NH 3 (g) ... The figure below represents the spontaneous reaction of H 2 (shaded spheres)with O 2 (unshaded spheres) ... -According to the diagram above, Free. Unlocked . Multiple Choice . Unlock ...
The diagram above represents a mixture of NO2(g) and N2O4(g) in a 1.0 L container at a given temperature. The two gases are in equilibrium according to the equation 2 NO2(g) ( N2O4(g). Which of the following must be true about the value of the equilibrium constant for the reaction at this temperature? A) K = 0. B) 0 < K < 1. C) K = 1. D) K > 1
Nov 15, 2021 · The diagram below represents a spontaneous reaction δg. The following diagram represents an imaginary two step mechanism. Entropy is the tendency of more concentrated energy to spread out into less concentrated forms. 2cog 2nog n2g. Is the reaction endothermic or. The diagram below represents a spontaneous reaction (ΔG°<0). Fill in tthe ...
The diagram below represents a spontaneous reaction (ΔG°<0). Fill in tthe blanks below. Learn this topic by watching Gibbs Free Energy Concept Videos. All Chemistry Practice Problems Gibbs Free Energy Practice Problems. Q. The reaction SO2 (g) + 2H2S (g) ⇌ 3S (s) + 2H2O (g) is the basis of a suggested method for removal of SO2 from power ...
Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and
Dec 03, 2021 · The diagram below represents a spontaneous reaction (δg (a) The reaction glyceraldehyde 3-phosphate 1,3-bisphosphoglycerate should be inhibited when levels of NADH fall. (b) The ΔG° for the oxidation of the aldehyde group on glyceraldehyde 3-phosphate to form a carboxylic acid is more negative than the ΔG° for ATP hydrolysis.
Nov 20, 2017 · The diagram below represents a spontaneous reaction δg molecules size. 2cog 2nog n2g. Chemical equation question with a diagram. For the other reactions there is a decrease in the number of moles of has so δs is negative. Answer to the diagram below represents a spontaneous reaction. Drag the labels to the correct bins.
Q: The one dimensional diffusion equation is given by au 2Ꭷ a2U ar2 (4) at This is to be solved numerically over the range 0 < x <1 to establish the temperature distrib A:See Answer Q: Question 5: (Total: 20 Marks) Consider the (2,1,2) convolutional code with: (1) = (101) g(2) = (011) A) Construct the encoder block diagram. (4 Marks) B) Draw the st A:See Answer
The image represents a spontaneous, gaseous reaction at a constant temperature T K. Predict whether ΔH, ΔS, and ΔG for this reaction are positive, negative, or zero. Acetylene, C2H2, can be converted to ethane, C2H6, by a process known as hydrogenation.
Throughout this topic I shall use the word "feasible", but if your examiners expect you to use the word "spontaneous", that is what you should do. Feasible changes and ΔG. I am dropping the "standard" symbol after ΔG from now on, because most of the time we shall be using it at non-standard temperatures.
A G vs. extent-of-reaction diagram for a non-spontaneous reaction can be interpreted in a similar way; the equilibrium composition will correspond to an extent of reaction greater than zero but less than 0.5. In this case, the minimum at reflects the increase in entropy when the reactants are "contaminated" by a small quantity of products.
5. Consider the reaction below and without reference to any data tables, draw the proper conclusion on which condition A-D best represents the reaction being spontaneous as written. CO 2 (g) + H 2O (g) --> HCOOH (l) ΔH = -150 kJ A. The reaction would be spontaneous at all temperatures. B.
A spontaneous reaction may involve an increase or decrease in enthalpy, it may involve an increase or decrease in entropy, but it will always involve a decrease in free energy that is a negative ΔG.
Base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Given the reaction: A + B --> C. a) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer.
Electrical Work From Spontaneous Oxidation-Reduction Reactions. The following rule can be used to predict whether an oxidation-reduction reaction should occur.Oxidation-reduction reactions should occur when they convert the stronger of a pair of oxidizing agents and the stronger of a pair of reducing agents into a weaker oxidizing agent and a weaker reducing agent.
The diagram below represents a spontaneous reaction δg. Q is the reaction quotient. Learn vocabulary terms and more with flashcards games and other study tools. A negative δg indicates a spontaneous reaction.
Nov 10, 2021 · The diagram below represents a spontaneous reaction (δg. The spontaneous redox reaction in a voltaic cell has _____ A) a negative value of Ecell and a negative value of ΔG. B) a positive value of Ecell and a positive value of ΔG. C) a negative value of Ecell and a positive value of ΔG. D) a positive value of Ecell and a negative value of ΔG.
When delta G is equal to zero, neither the forward nor the reverse reaction is spontaneous. The correct option is A. A system at equilibrium has its delta G equals to zero, at this point, the free energy change is neither positive nor negative; the reaction is at equilibrium and both the forward and the reverse reactions are not spontaneous.
The Pt(s) is an inert electrode in contact with the MnO 4-(aq), Mn2+(aq) solution.This half is on the left so is the oxidation half cell, so the half cell reaction is reversed.
spontaneous. 5. Is this reaction always spontaneous? If not, determine at what temperatures it changes from spontaneous to non-spontaneous. With a -∆H°, and +∆S°, this reaction will be spontaneous under all conditions. 6. For the following reaction: 2Mg(s) + O 2 (g) MgO(s) , ΔHo rxn = -1202 kJ/mol; ΔSo rxn = -217 J/mol∙K

The diagram represents a spontaneous reaction. use the diagram to answer the questions below.a. is the reaction endothermic or exothermic?b. what is the activation energy of the reaction?








/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)








0 Response to "34 the diagram below represents a spontaneous reaction (δg"
Post a Comment