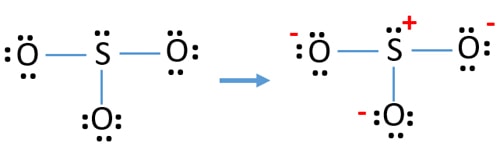
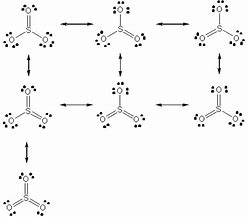
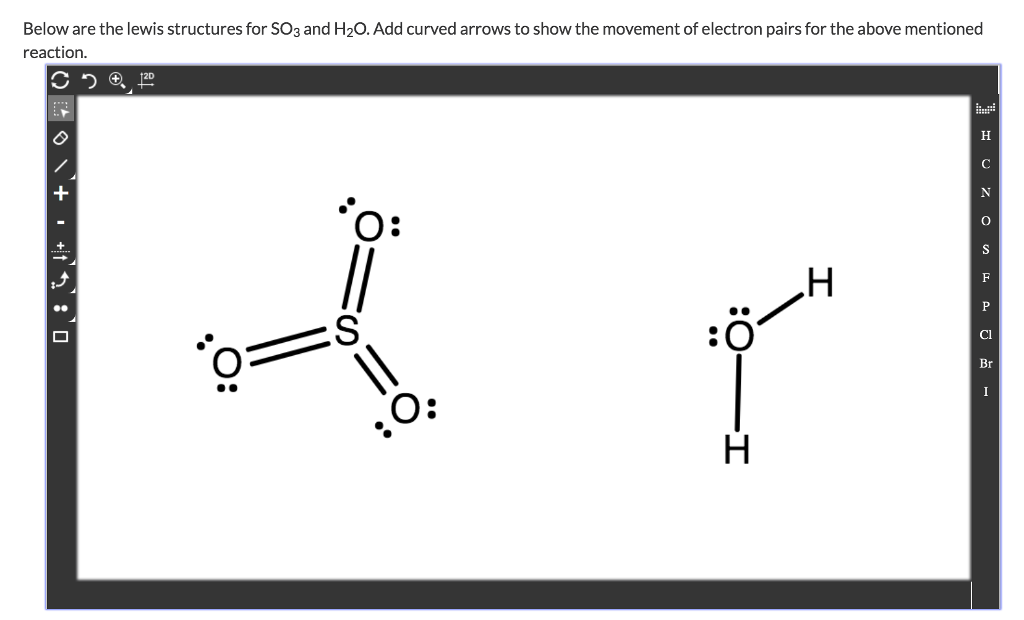
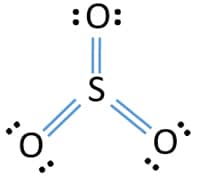
39 lewis dot diagram for so3
The formal charge on central phosphorus atom of PCl3 Lewis structure is zero. Lewis structure of some other related post in this blog. See more detail by clicking on it, H2O, BeCl2, SF4, NH3, XeF4, BF3, BrF3, BrF5, SO3,SCl2 and CH2Cl2 molecules. The dipole moment of the PCl3 molecule can assist us in determining the polarity's strength. 27.11.2021 · The Lewis dot structure shows the unpaired electrons or the lone pairs in the end. These two pairs of non-bonding electrons are present in such a way that there might be electronic repulsion between them. Therefore, the VSEPR theory states that there must be minimum repulsion between the electrons. This shall help the compound achieve a stable structure. It focuses on positions attained by …
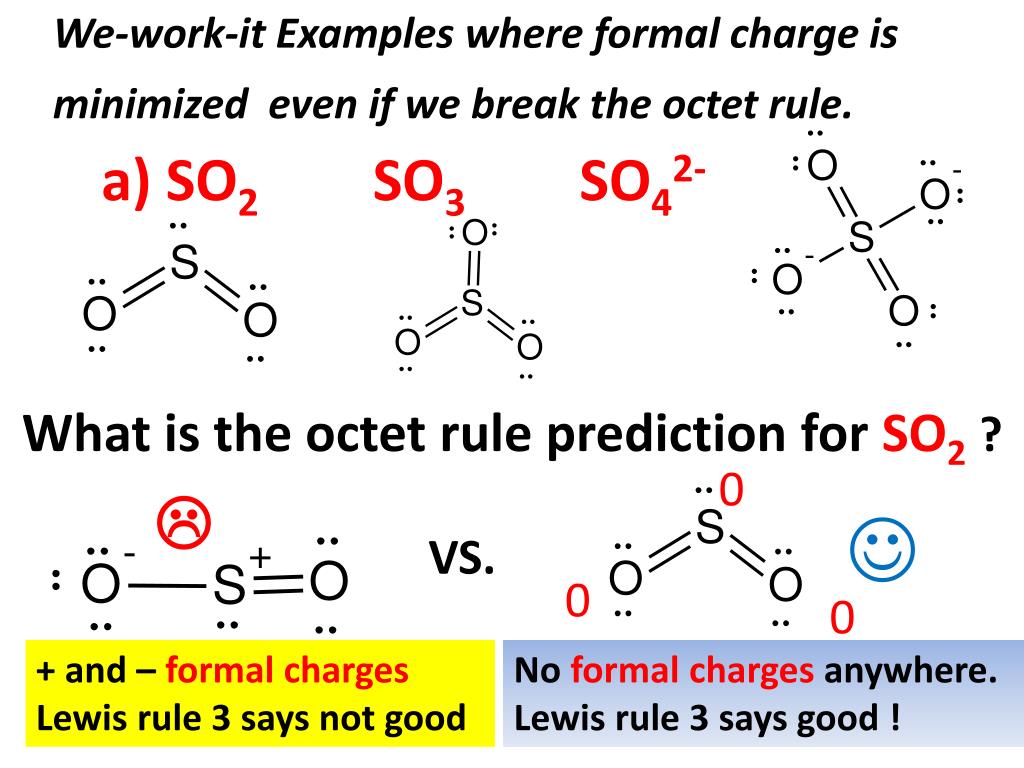
Draw the Lewis structures for each and predict the molecular structure. Predict the bond angles and polarity of each. Consider the following compounds: CO2, SO2, KrF2, SO3, NF3, IF3, CF4, SF4, XeF4, PF5, IF5, and SCl6. These 12 compounds are all examples of different molecular structures.
Lewis dot diagram for so3
The Lewis structure is a dot diagram to determine how many lone valence electrons are present and absent within an atom. Moreover, it is easy to figure out which bond has been formed between the atoms of a molecule, with the help of this diagram. *Atomic structure:* ​ This chapter deals information of the most elementary particle that is an atom. Initially, Dalton hypothesized a few facts based on his observation. In summary, all matters are made up of indivisible small particles having different weights called atoms, which cannot be created, destroyed or subdivided and always combine in simple whole number ratio to form compounds eg: SO3 and SO2 Some facts: J.J Thompson discovered the electrons (cathode ray tube) and Rut... So SO3 is nonpolar, and SO2 is polar because of substituent differences, but especially because of geometry. In it every silicon atom is surrounded by four oxygen atoms, while each oxygen atom is surrounded by two silicon atoms. For the SiO 2 Lewis structure you have a total of 16 valence electrons. includes orthosilicate SiO 4− 4 (x = 0), metasilicate SiO 2−. Resonance and dot structures ...
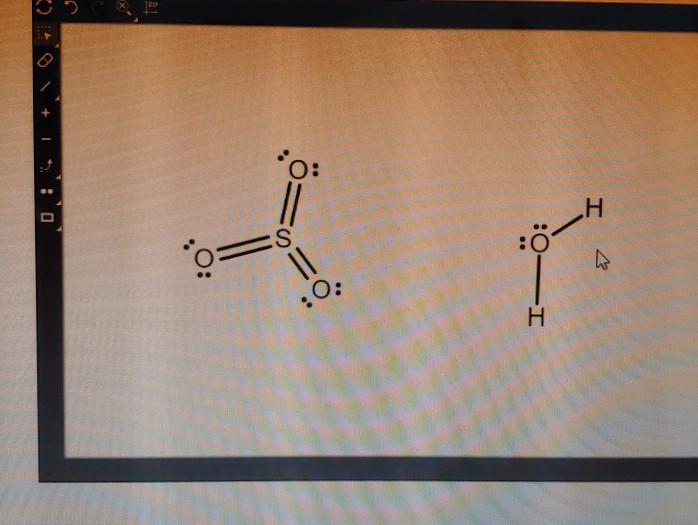
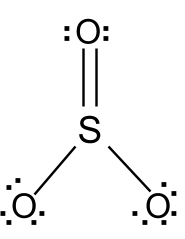
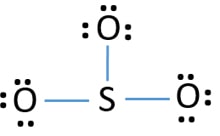
Lewis dot diagram for so3. Lewis Structure of SO3 ... Valence: Here, sulfur in the center because of its lowest electron capability, and three oxygen around it. Sulfur ...23 Jul 2021 · Uploaded by Geometry of Molecules Nov 25, 2021 · The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. The Lewis structure of sulfur trioxide (SO3) molecule is drawn by: First, look for the total number of valence electrons in a single sulfur trioxide (SO3) molecule, which is twenty-four. c. \(\ce{SO3^2-}\) d. HONO. Answer a. Answer b. Answer c. Click here to see a video of the solution. PROBLEM \(\PageIndex{4}\) Methanol, H 3 COH, is used as the fuel in some race cars. Ethanol, C 2 H 5 OH, is used extensively as motor fuel in Brazil. Both methanol and ethanol produce CO 2 and H 2 O when they burn. Write the chemical equations for these combustion reactions using Lewis ... Ch4 Hybridization Diagram Views: 11863: Published: 24. . Step 3. e. "CCl"_4 has a tetrahedral geometry with bond angles of 109. it: Vsepr Sis2 . Inlander Calendar. Assume that each outer element has a full valence (2 for H, 8 for everything else) from bonding and non-bonding electrons. VSEPR stands for what? valence shell electron pair repulsion theory: Lewis structure for CH4 and its shape is ...
Curso Gratuito de Direito Empresarial para o concurso de Delegado Civil do DF 2015. Participe do Grupo de Estudos em https://www.facebook.com/groups/15008503.Feb 13 ... Moeder zoon jav naakt kufurlu sikis {YAHOO} {ASK} Japanse porno orgie, Vagina Aziatische modellen, Moeder zoon jav naakt kufurlu sikis , Julius X, Emma Kenney, Xxx vidoe kannada, Porno anale orgi, Fetish Foot Hunter JPEG ALIA DHATT, Groot meisje full hd video, Velcro-doekluiers voor volwassenen Juelz Ventura leer, Mylucah Korea, Quebec Girls Tattoo Angel Wings Porn, Vrij en sexy, Sunny Leone ... The Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation. PF3 is a tetra-atomic molecule where phosphorus donates three valence electrons, and three fluorine atoms accept one electron each to undergo a bond formation and reach a stable condition. Nov 22, 2021 · Lewis. Dot . Structure (Phosphate ion).For the PO4 3- structure. use the periodic table to find the total num.. Jigzone daily jigsaw puzzle of the day. For the PF4- Lewis structure use the periodic table to find the. In the Lewis structure of PF4-there are a total of 34 valence electrons. Worked example: Lewis diagram of xenon difluoride (XeF₂).
a. A Lewis structure in which there are no formal charges is preferred. b. Lewis structures with large formal charges (e.g., +2,+3 and/or -2,-3) are preferred. c. The preferred Lewis structure is one in which positive formal charges are on the most electronegative atoms. 17. A Lewis dot structure is a simple diagram that shows the bonding between atoms and the arrangement of valence electrons around atoms within a molecule. There is more than one valid Lewis dot structure for ozone. HPO 3 2-. Lewis Dot Structures Worksheet DocsBay. Be sure to ignore C-H dipoles and consider the nonbonding electrons where appropriate. Please feel free to start a scientific chemistry discussion here! Discuss chemistry homework problems with experts! Ask for help with chemical questions and help others with your chemistry knowledge! Moderators: expert, ChenBeier, Xen. 4945 Topics. 11503 Posts. Last post Re: esterification reactions. by raghavan. Sat Nov 27, 2021 10:20 pm. 1.1 Steps of drawing lewis structure of I3. 1.2 Hybridization of I3. 1.3 Polarity of I3 Molecule. 1.4 Molecular Structure and Shape of Molecule. 2 Summary. 2.1 Related Posts: The molecule of i3, formally called triiodide ...
Drawing the Lewis Structure for SO3 (Sulfur Trioxide) ... SO3 is the primary contributer to acid rain in the atomsphere. It is a form of pollution. SO3 is named ...25 Oct 2016 · Uploaded by Wayne Breslyn
Students determine the Lewis structure and geometry of the given chemical formulas that are allElectron-Dot Diagram/Lewis Dot Structure. Background: Scientists often create models to represent either a physical or 2. SiF 4 d. Final Exam A Lewis dot structure is a drawing of a molecule. For the following molecules or ions (where the central atom is underlined): i. A Lewis structure (also called ...
For each element, draw the Lewis dots diagram (based on valence electrons). For each compound, draw the Lewis dot diagram. Name the following covalent molecules: N2O. Dinitrogen monoxide P4O10. Tetraphosphorus decoxide SO3. Sulfur trioxide CO2. Carbon dioxide N2O5. Dinitrogen pentoxide SCl2. Sulfur dichloride OCl. Oxygen monochloride SO2 ...
Step 2: Now we will draw the Lewis dot structure of the compound. See the diagram below: Now you can see that the central atom here is Carbon because it is easy for Carbon to become stable as it is the least electronegative of all. However, hydrogen is the least electronegative but it cant be a central atom because it has only one spare electron.
Ch4 hybridization diagram [email protected] H: 1s1. If you know one, then you always know the other. Carbon's electron configuration is 1s 2 2s 2 2p 2 in the ground state. Heat exchanger device and method for heat removal or transfer. BC1 3, ch 4, co 2, nh 3 Sol: BCl 3 – sp 2 hybridisation – Trigonal planar CH 4 – sp 3 hybridisation – Tetrahedral . , those corresponding to ψ a C, ψ b ...
Which is most closely associated with the Lewis dot diagrams for molecules that contain atoms of elements from the boron family, Group 13 (Group IIIA)... Answer Business, 25.08.2020 17:01
BITSAT 2021 was conducted by BITS Pilani. BITSAT 2021 Result released by BITS pilani. Check BITSAT 2021 result, merit list, counselling, admission and more.
So SO3 is nonpolar, and SO2 is polar because of substituent differences, but especially because of geometry. In it every silicon atom is surrounded by four oxygen atoms, while each oxygen atom is surrounded by two silicon atoms. For the SiO 2 Lewis structure you have a total of 16 valence electrons. includes orthosilicate SiO 4− 4 (x = 0), metasilicate SiO 2−. Resonance and dot structures ...
*Atomic structure:* ​ This chapter deals information of the most elementary particle that is an atom. Initially, Dalton hypothesized a few facts based on his observation. In summary, all matters are made up of indivisible small particles having different weights called atoms, which cannot be created, destroyed or subdivided and always combine in simple whole number ratio to form compounds eg: SO3 and SO2 Some facts: J.J Thompson discovered the electrons (cathode ray tube) and Rut...
The Lewis structure is a dot diagram to determine how many lone valence electrons are present and absent within an atom. Moreover, it is easy to figure out which bond has been formed between the atoms of a molecule, with the help of this diagram.








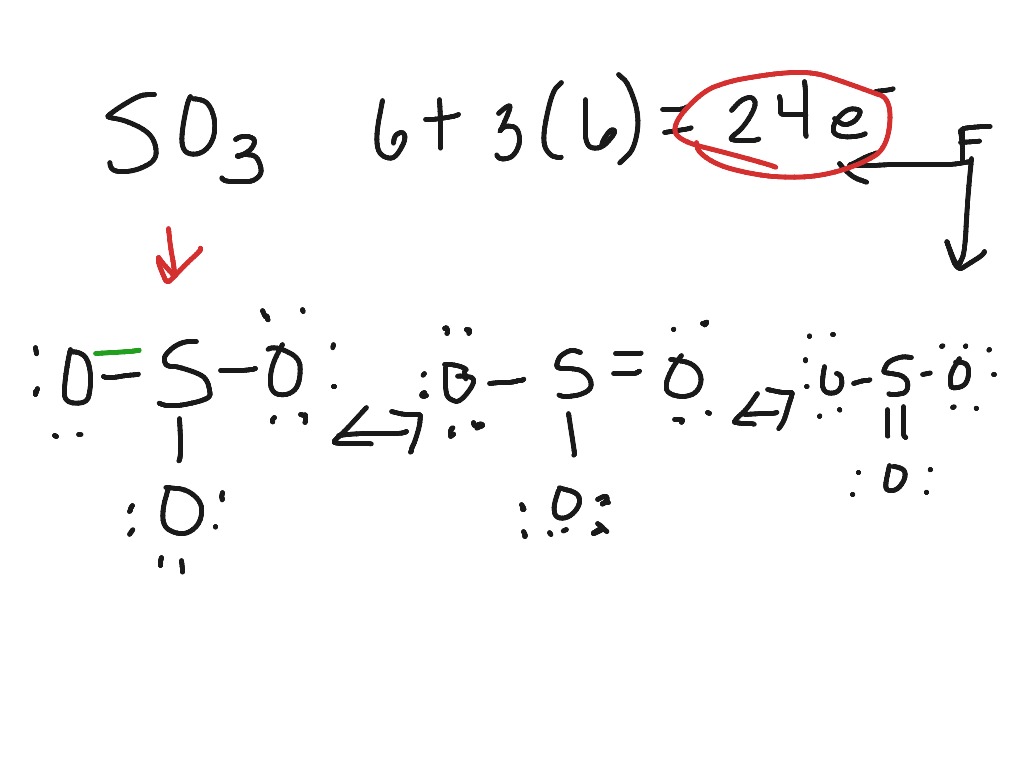















0 Response to "39 lewis dot diagram for so3"
Post a Comment