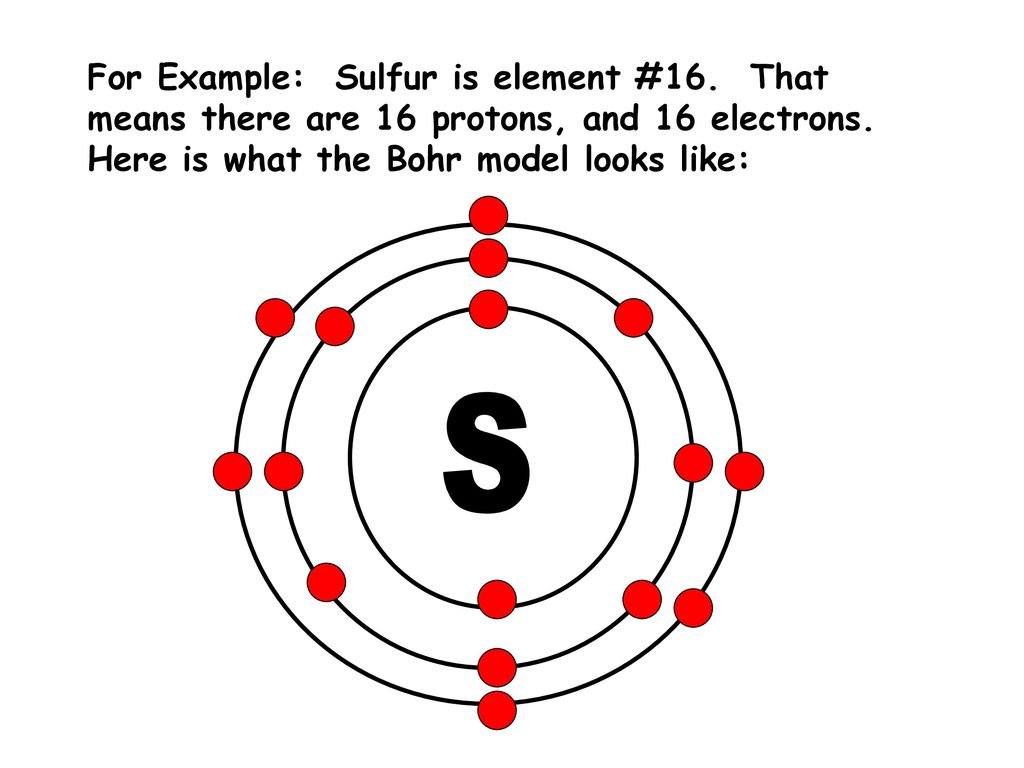
39 lewis dot diagram for sulfur
A step-by-step explanation of how to draw the SI2 Lewis Dot Structure.For the SI2 structure use the periodic table to find the total number of valence electr...
23 Jul 2018 — Sulfur is in group 16/VIA, so its atoms have six valence electrons. The Lewis dot symbol for an element represents the valence electrons as ...1 answer · Refer to the explanation. Explanation: Sulfur is in group 16/VIA, so its atoms have six valence electrons. The Lewis dot symbol for an element represents ...
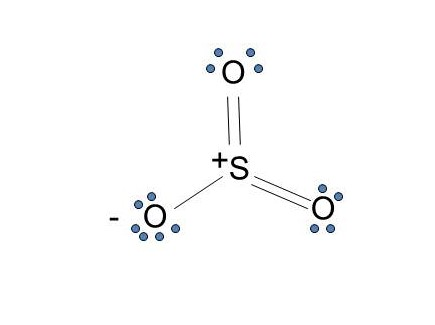
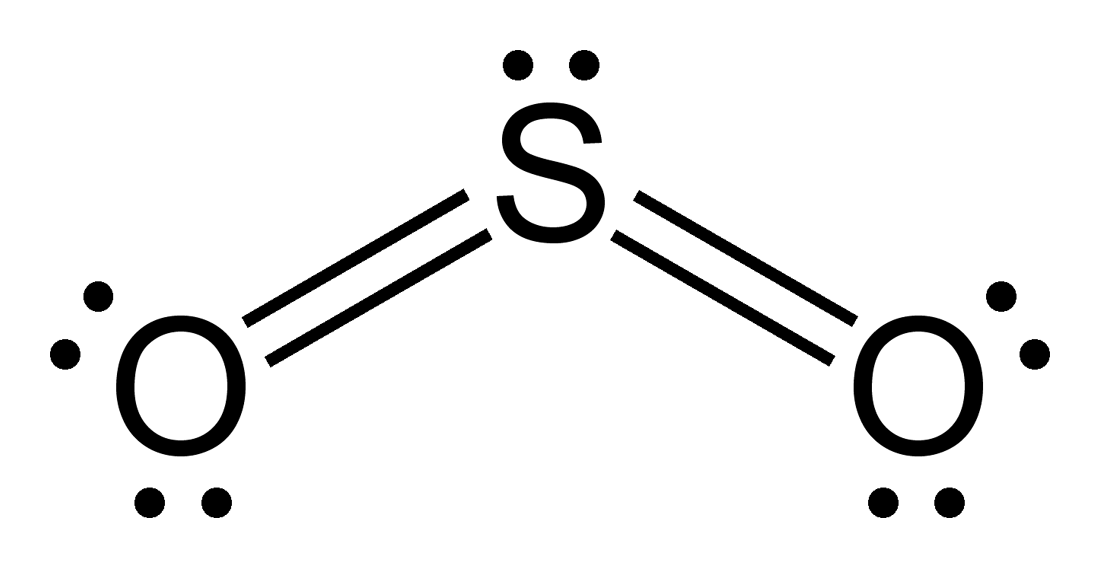
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access . Drawing the Lewis Structure for SO 2.

Lewis dot diagram for sulfur
Lewis Dot Diagram For So4 2. Simple procedure for drawing covalent Lewis structures - Lewis dot of the sulfate ion SO, best lewis structure for so, electron bonding. Viewing Notes: The Lewis structure for SO is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis.
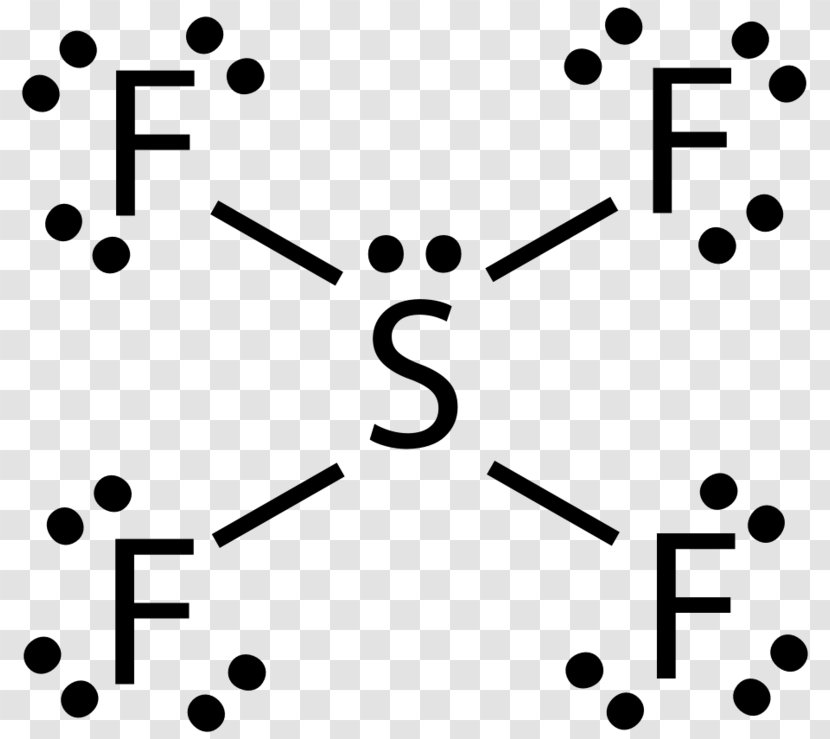
SF6 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram SF6 or sulfur hexafluoride is an inorganic and one of the most stable gases that are known in chemistry. This gas has more density than air. The general identification of the gas can't be done because it is odorless and colorless in nature.
The Sulfur Dioxide which is also known as Sulphur Dioxide is the entity of a bond between Sulfur and Oxygen atoms. It is known as a formula written as SO2. Here we will provide an explanation of SO2 molecular geometry, SO2 electron geometry, SO2 bond angle, and SO2 Lewis structure.
Lewis dot diagram for sulfur.
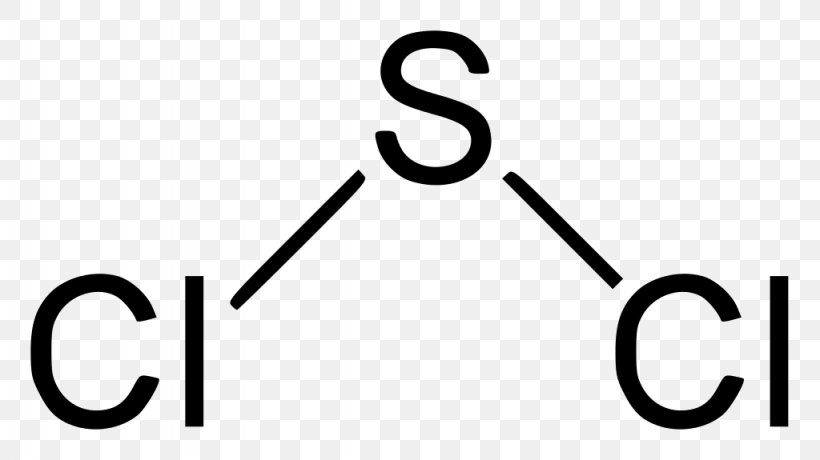
SCl2 lewis structure contains one sulfur and two chlorine atom. Sulfur being the less electronegative atom than chlorine atom is placed at the center in lewis’s diagram and chlorine is spaced evenly around it. There is two lone pair present on the central atom and this central atom attached to two bonded pair in SCl2 lewis structure.
The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur.
Draw the Lewis Dot Structure. Notes: Scientists use. Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons.
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
One point is earned for the molecular geometry consistent with the Lewis diagram in part (a). (c) In the SO 2 molecule, both of the bonds between sulfur and oxygen have the same length. Explain this observation, supporting your explanation by drawing in the box below a Lewis electron-dot diagram (or diagrams) for the SO 2 molecule.
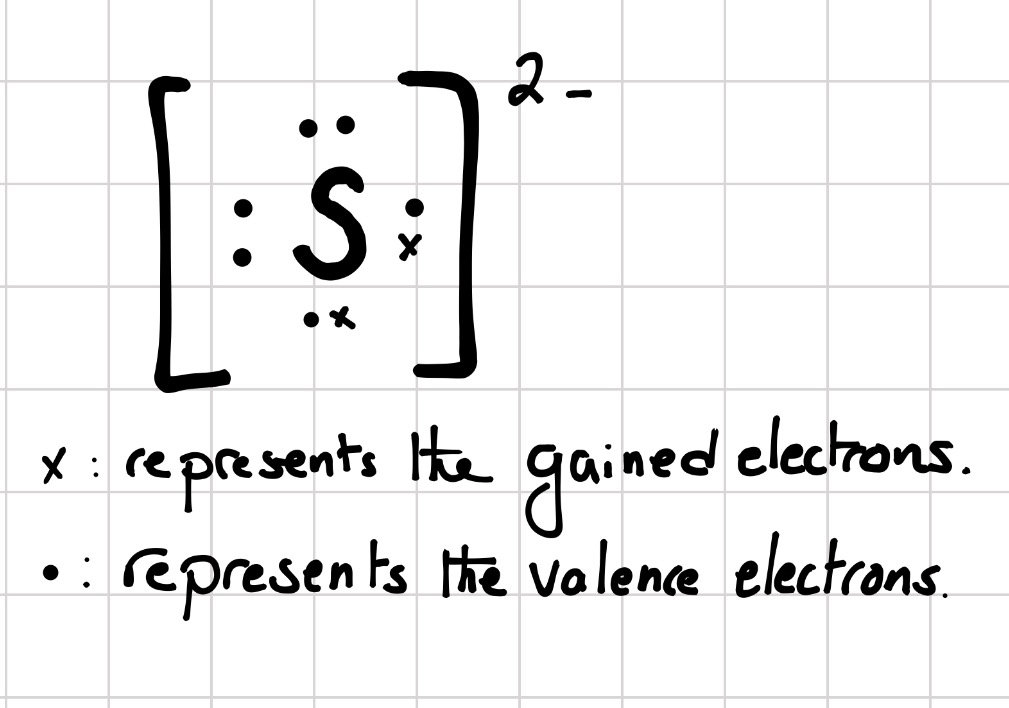
Now let us try Lewis dot structure of Sulfide ion ( S2-). Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom. Likewise, why does sulfur not follow the octet rule?
Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules. 3. Determine the electron and molecular geometry of …
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Electrons exist outside of an atom's nucleus and are found in principal energy levels that contain only up to a specific number of electrons.
Lewis Dot Diagram For Sulfur, Potassium Sulfide Facts, Formula, Properties, Uses, Calcium Sulfide YouTube, Lewis Dot Structure of SO3 (Sulfur Trioxide) YouTube, SO3 Hybridization: Hybrid Orbitals for SO3 (sulfur
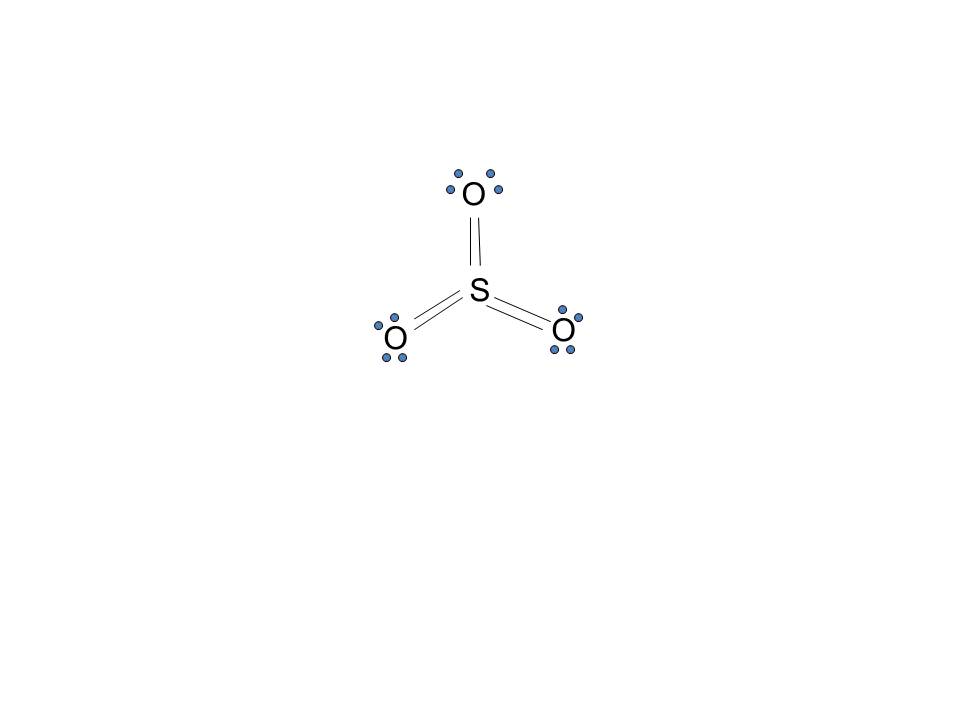
Lewis structure of SO3 The sulfur trioxide is a tetra atom chemical molecule whereby both the sulfur and also three oxygen molecules bond with an equal variety of valence electrons. The chart is attracted showing dots the valence electrons roughly the prize of both sulfur and also oxygen atoms through lines predicting link formation.
Drawing the Lewis Structure for SOCl 2. Viewing Notes: The Lewis structure for SOCl 2 requires you to place more than 8 valence electrons on Sulfur (S).; You might think you've got the correct Lewis structure for SOCl 2 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons.
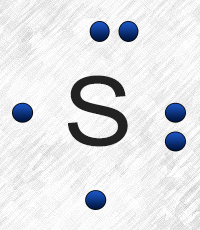
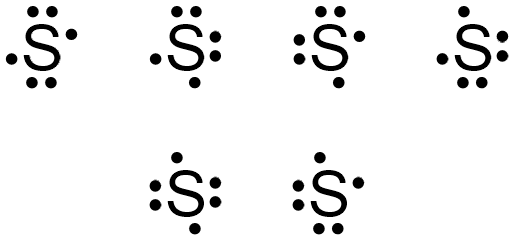
Sulfur (S) has six valence electrons. A Lewis dot structure of around 'S' would have two dots on two sides, and one single on each of the remaining. It would look be drawn as: .. .S : .
• (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding.
Lewis dot structure will have 4 paired dots around Sulfur atom.For atoms and monoatomic ions, step one is sufficient to get the correct Lewis structure. Lewis dot structures for Polyatomic ions and molecules : However for molecules and polyatomic ions we need to consider many more factors before drawing a correct Lewis dot structure.
A Lewis Dot Structure is the diagrammatic representation of the bonding between the atoms of a molecule and the lone pair of electrons present in it. It is also known as electron dot structure/ Lewis dot diagram, Lewis dot formulas, Electron dot structure, or Lewis Electron dot structure (LEDs), respectively.
For sulfur atom ⇒ Valence electrons of sulfur = 6 ⇒ Lone pair electrons on sulfur = 4 ⇒ Bonding electrons around sulfur (2 single bonds) = 4 ∴ (6 - 4 - 4/2) = 0 formal charge on the sulfur central atom. So, this is our most stable and appropriate lewis dot structure or electron dot structure of SBr2.
For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s 2 3d 6) is as follows:. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration.
How to draw the Lewis Structure of SO2 - with explanationCheck me out: http://www.chemistnate.com
Electron Dot Diagrams and Lewis Structures. STUDY. Flashcards. Learn. Write. Spell. Test. PLAY. Match. Gravity. Created by. Cristy_Duran3. Terms in this set (18) ... electron dot diagram for Boron. electron dot diagram for Sulfur. electron dot diagram for Carbon. electron dot diagram for Phosphorus. Lewis structure for PCl₃ ...
Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.
A Lewis electron dot diagram ... Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. ...
Sulfur Tetrafluoride has 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the central atom in its Lewis structure. There are three lone pairs on each fluorine atom. It has a molecular geometry of the formula AX4E; it forms a see-saw shape and has a trigonal bipyramidal molecular geometry. SF4 has ...
A step-by-step explanation of how to draw the Lewis dot structure for S (Sulfur). I show you where Sulfur is on the periodic table and how to determine how ...
Lewis structures are not a good model for this molecule: Oxygen and sulfur have 6 valence shell electrons. In total 24 electrons to be drawn ( or 12 pairs. ) 5 pairs are already taken for bonding as displayed above. So 7 pairs left. 4 pairs on the oxygen 2 on the sulfurs.
Sulfur And Chlorine Lewis Dot Diagram, Show The Orbital Filling Diagram For Br Bromine Wiring, Lewis Dot Diagram For Potassium, How the ionic bond forms in Lithium Sulfide (Li2S) YouTube, Valence Shell Electron Pair Repulsion
A Lewis dot structure is a drawing of a molecule. The drawing only “works” f0r stable molecules that actually exist. So it’s a nice tool to explore how atoms bond into more complex substances. A Lewis dot structure is also called a Lewis structure, a Lewis dot diagram, an electron dot structure, or a dot diagram.
Lewis Dot Diagram For Sulfur – Atkinsjewelry from hi-static.z-dn.net Metallic elements, to the left of the staircase dividing line, tend to loose one or more electrons and form ions. In total 24 electrons to be drawn ( or 12 pairs. The total number of valence electrons for s is 6 (sulfur is also in the 6th column of the periodic table).
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access to the . Here are the steps I follow when drawing a Lewis structure.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) or dots ( ) but a lone pair of two electrons is shown by dots [ ].
A Lewis electron dot diagram A representation of the valence electrons of an atom that uses dots around the symbol of the element. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
Sf4 2 lewis structure. C of sulphur is. Nov 01, 2021 · To sketch the AsF3 Lewis structure by following these instructions: Step-1: AsF3 Lewis dot Structure by counting valence electrons on the Arsenic atom. Sundin Home | [email protected
After that I draw the Lewis dot structure for Sulfur (S). Note: Sulfur is in Group 16 (sometimes called Group VI or 6A). Since it is in Group 6 it will have 6 valence electrons. When you draw the Lewis structure for Sulfur you'll put six 'dots' or valance electrons around the element symbol (S).
Again, it does not matter on which sides of the symbol the electron dots are positioned. For carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell. As usual, we will draw two dots together on one side, to represent the 2s electrons. However, conventionally, we draw the dots for the two p electrons on different sides. . As such, the …



















0 Response to "39 lewis dot diagram for sulfur"
Post a Comment