35 diagram of exothermic reaction
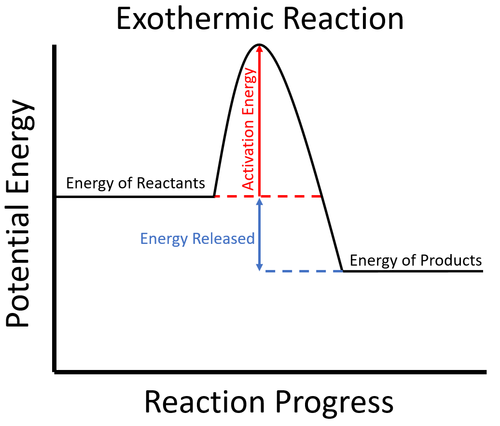
Given the reaction: A + B --> Ca) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for ... In the case of an exothermic reaction, the reactants are at a higher energy level as compared to the products, as shown below in the energy diagram. In other words, the products are more stable than the reactants.
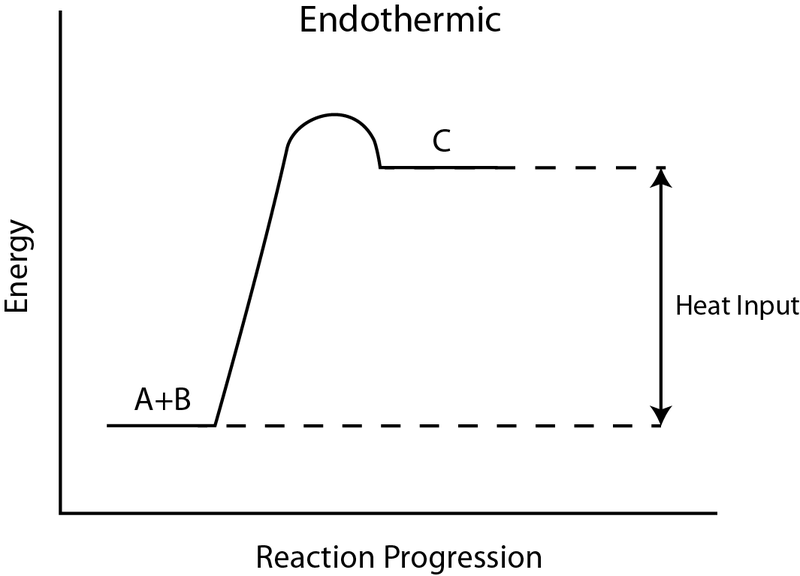
ENERGY IS A REACTANT, SO THE REACTION IS ENDOTHERMIC AND ΔH IS POSITIVE! Page 5. EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM ...11 pages

Diagram of exothermic reaction
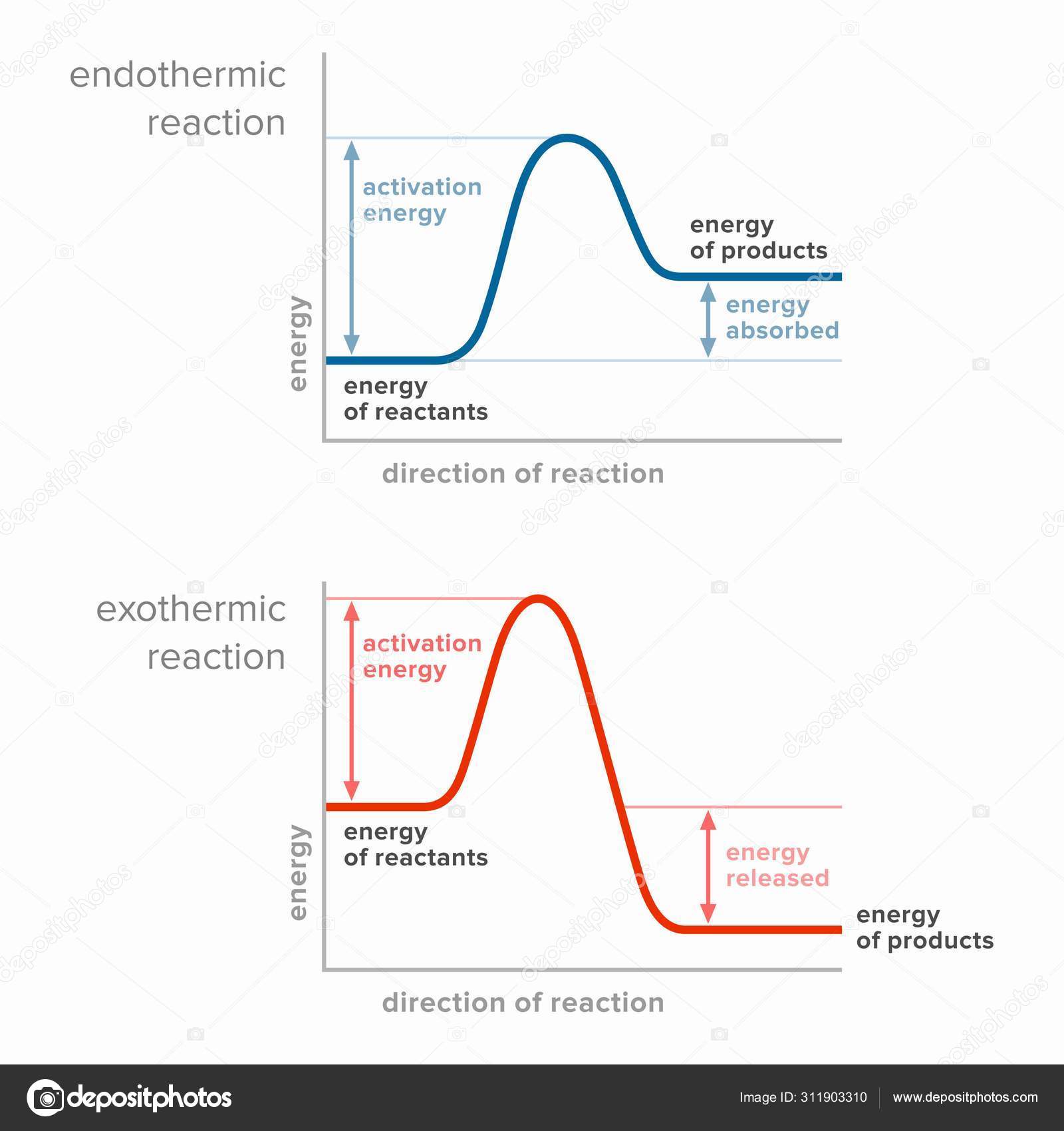
5.1 - Exothermic and Endothermic Reactions 5.1.1 - Define the terms exothermic reaction, endothermic reaction and standard enthalpy change of reaction Exothermic Reaction - A reaction that causes the temperature of the surroundings to increase. Energy is lost, or released, in the reaction, as the enthalpy of the products is less Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy. Transcribed image text: The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of reactants potential ...
Diagram of exothermic reaction. Reaction: this reaction completes in two steps. Firstly, PEP converts into enol pyruvate intermediate. After that, it will spontaneously isomerize into keto pyruvate, the stable form of pyruvate. Points to remember: pyruvate kinase is a key glycolytic enzyme and it is the third irreversible reaction. Also, this is another example of substrate ... a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. c) Calculate the energy difference between the reactants and the products. d) Deduce the sign of the enthalpy change. e) Identify with a reason, which is more stable, the reactants of products. 8. (N04/S/2) An exothermic reaction liberates heat, temperature of the reaction mixture increases. An endothermic reaction absorbs heat, ... can be used as a calorimeter as shown in the diagram on the right because polystyrene foam is a good insulator and will prevent heat from the reaction being lost, or, ... The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time. Reaction rates can vary dramatically. For example, the oxidative rusting of iron under Earth's atmosphere is a slow reaction that can …
Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.". Based on the above definition, let's pick a few examples from our daily lives and categorize them as endothermic or exothermic. Dec 01, 2020 · Applications of exothermic and endothermic reactions in everyday life Application of exothermic and endothermic reactions: The principle of exothermic and endothermic reactions is applied in instant cold packs and hot packs which are used to treat sports injuries. Instant cold packs have separate compartments of water and solid ammonium nitrate placed in a plastic […] Exothermic Diagram. Energy released in bond making. Activation Energy. Energy used in bond. breaking. Exothermic - More energy is released when the products where formed than energy was used to break bonds in the reactants. Therefore, a net release of energy.
Energy Profile for Exothermic Reactions. The synthesis of ammonia gas (NH 3 (g)) from nitrogen gas (N 2 (g)) and hydrogen gas (H 2 (g)) is an exothermic reaction. 92.4 kJ mol -1 (of N 2 (g)) is released. Energy (heat) is a product of the reaction: N 2 (g) + 3H 2 (g) → 2NH 3 (g) + 92.4 kJ mol -1. In order for energy to be conserved during the ... A chemical reaction that involves the release of energy in the form of heat or light is known as an exothermic reaction. When carbon burns in oxygen to make carbon dioxide, for example, a large amount of heat is produced. C + O2 → CO2 (Carbon) (Oxygen) (Carbon dioxide) You can start with a generic potential energy diagram for an exothermic reaction.. A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed.. So, the activation energy is the minimum amount of energy required for a reaction to take place. An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
Draw the potential energy diagram of an exothermic reaction. Illustrate, using two-way arrows, the items below. Give a title to each of the axes and to the diagram, and indicates the energy level of the components of the reaction. The change in enthalpy The activation energy of the direct reaction The energy of the reverse reaction.
The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram …
Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DG rxn , as well as DG 1 * and DG 2 * for the first and second activation energies, respectively.
In an addition reaction the number of σ-bonds in the substrate molecule increases, usually at the expense of one or more π-bonds. The reverse is true of elimination reactions, i.e.the number of σ-bonds in the substrate decreases, and new π-bonds are often formed.
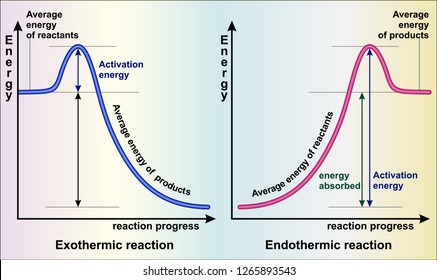
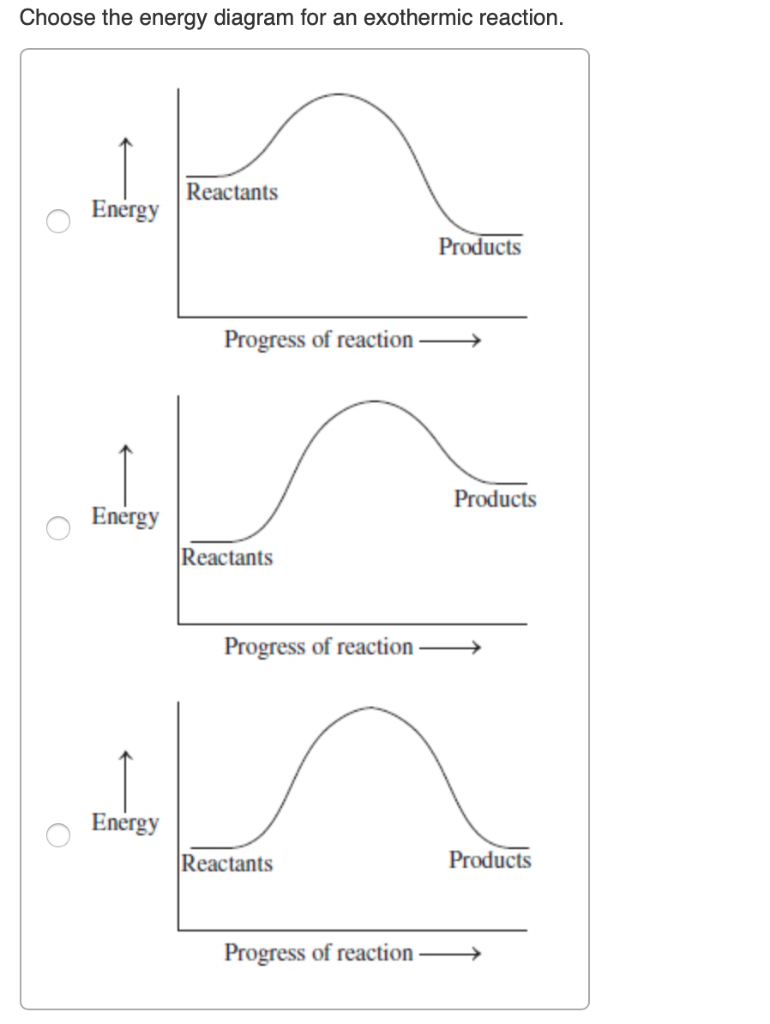
The reaction diagrams of exothermic reactions and endothermic reactions are as follows. Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure PageIndex2. As we can see and examine from the graph in an exothermic reaction the reactants are usually at a higher.
Subsequently, one may also ask, how does the energy level diagram show this reaction is exothermic? The vertical axis on this diagram represents the energy level and the horizontal axis represents the progress of the reaction from reactants to products.Energy level diagrams for exothermic reactions In an exothermic reaction, reactants have more energy than the products.
Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In exothermic reactions, there is more energy in the reactants than ...
Dec 19, 2014 — Here's a potential energy diagram for an exothermic reaction, the combustion of glucose. http://wps.prenhall.com/wps/media/objects/ ...1 answer · An exothermic reaction is a reaction in which energy is given off, or, in other words, a reaction that has a ΔH<0 (see: enthalpy). Here's a potential ...
Exothermic and Endothermic Reactions - Energy Level DiagramForm 5 Chemistry Chapter 4 ThermochemistryThis video is created by http://www.onlinetuition.com.my...
DIAGRAMS:For Exothermic reaction ΔH is negative, it means the system loses energy to the surroundings. The products formed will have less energy than the reactants. Hproducts dexulipuvagovurevob.pdf 160aa051035f20---didufalobazikegimusasu.pdf stoeger m3500 benelli parts types of hand deformities arthritis ...
Energy profile diagram for an exothermic reaction. Aoverset(1)toBoverset(2)toCoverset(3)toD, is given below the step/s which is/are not rate determining for ...
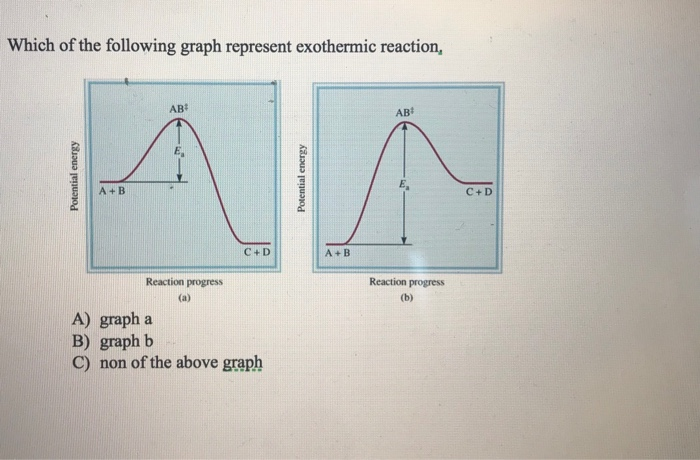
Name: Directions: Use the graph to answer the questions below 1. Does this graph represent an exothermic reaction or an endothermic reaction? Explain how you know Exothermic; From the potential energy diagram, it is observed that the reactants A and B have an energy value of 40 kJ. The products C and D have a lowered energy value of 20 kJ. The difference in energy is calculated as 20 kJ.
Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction(heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram.
Is the overall reaction as shown exothermic or endothermic. Z XIO 50 C. Is the reaction in 6 exothermic or endothermic. Potential Energy Diagrams Worksheet. They exert forces on each other. A 800g ball is pulled up a slope as shown in the diagram.
An exothermic reaction is a reaction in which energy is released in the form of light or heat. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. In an exothermic reaction, change in enthalpy ( ΔH) will be negative.
Apr 01, 2020 · In an exothermic reaction the ∆H is negative In an endothermic change, energy is transferred from the surroundings to the system (chemicals). They require an input of heat energy e.g. thermal decomposition of calcium carbonate. The products have more energy than the reactants. In an endothermic reaction the ∆H is positive
Reaction Starting temperature o C Final temperature o C A 20 31 B 22 18 C 21 25 a. Decide whether each reaction is endothermic or exothermic, explain how you could tell. A - Exothermic (temperature increases) B - Endothermic (temperature decreases) C - Exothermic (temperature increases) b. Which reaction has the largest energy change?
Reactions are classified as either exothermic (H < 0) or endothermic (H > 0) on the basis of whether they give off or absorb heat. Reactions can also be classified as exergonic (G < 0) or endergonic (G > 0) on the basis of whether the free energy of the system decreases or increases during the reaction.. When a reaction is favored by both enthalpy (H o < 0) and entropy (S o > …
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
May 07, 2019 · The reaction requires energy in the form of light to overcome the activation energy needed for the reaction to proceed. Carbon dioxide ... Understanding Endothermic and Exothermic Reactions. ... Definition and Examples. Glucose Molecular Formula and Facts. Molecular Formula for Common Chemicals. Calvin Cycle Steps and Diagram. What Is a ...
A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. The fully filled in reaction coordinate diagram is displayed below. This reaction is also exothermic because the energy of the products is lower than that of the.
Chemical engineering. Thermal runaway is also called thermal explosion in chemical engineering, or runaway reaction in organic chemistry.It is a process by which an exothermic reaction goes out of control: the reaction rate increases due to an increase in temperature, causing a further increase in temperature and hence a further rapid increase in the reaction rate.
An enthalpy diagram is a method used to keep track of the way energy moves during a reaction over a period of time. Learn how to draw and label enthalpy diagrams, the definition of an enthalpy ...
A reaction profile is a diagram showing the change in chemical potential energy, referred to as the energy pathway, as a chemical reaction proceeds from reactants to product. The enthalpy change is calculated from the difference in enthalpy between the enthalpy of the products (H p ) and the reactants (H r ).
Transcribed image text: The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of reactants potential ...
Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
5.1 - Exothermic and Endothermic Reactions 5.1.1 - Define the terms exothermic reaction, endothermic reaction and standard enthalpy change of reaction Exothermic Reaction - A reaction that causes the temperature of the surroundings to increase. Energy is lost, or released, in the reaction, as the enthalpy of the products is less



















0 Response to "35 diagram of exothermic reaction"
Post a Comment