36 lewis diagram for n2
The second modification made to the ideal gas law accounts for interaction between molecules of the gas. The Van der Waals equation includes intermolecular interaction by adding to the observed pressure P in the equation of state a term of the form /, where a is a constant whose value depends on the gas. Lewis structure of N2 A Lewis Structure is a very simple representation of the valence, or outermost, electrons in a molecule. It does not explain the geometry of the molecule, but it is a step forward in approaching the geography. But to find out if N2 is polar or nonpolar, the Lewis Structure can reveal the best electron makeup of the molecule.
Jurnal Rekursif, Vol. 4 No. 2 Juni 2016, ISSN 2303-0755 176 ejournal.unib.ac.id RANCANG BANGUN APLIKASI TABEL PERIODIK UNSUR DAN PERUMUSAN
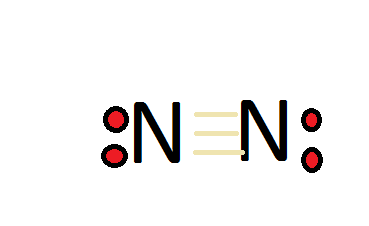
Lewis diagram for n2
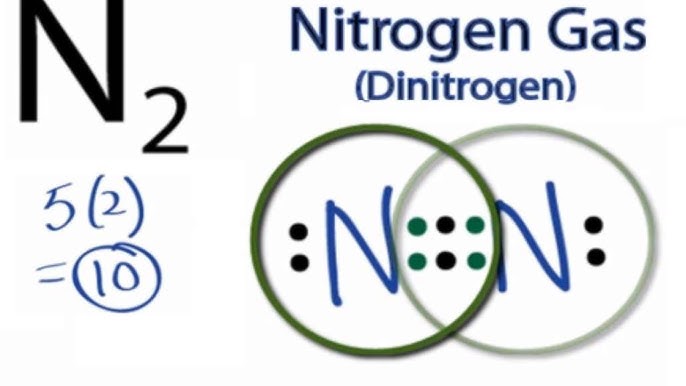
1. Complete the Lewis dot structure(s) for 2. Complete the Lewis dot sttllcture(s for 3. Complete the Lewis dot structure(s) for COC12 4. Complete the Lewis dot structure(s) for NOY :8-N-ë 5, Complete the Lewis dot structure(s) for C103 STOP Your group will check your answers with the instructor before moving on. Model VI: Multi-Centered Molecules Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. N2 Lewis Structure Setup It's easiest to think in terms of dots to make the N 2 Lewis structure. Nitrogen needs to bond three times, shown as the lone dots on the left, right and bottom of the N atoms in the below diagram. There is also a pair of dots, representing two more electrons, that won't bond, on top of each N.
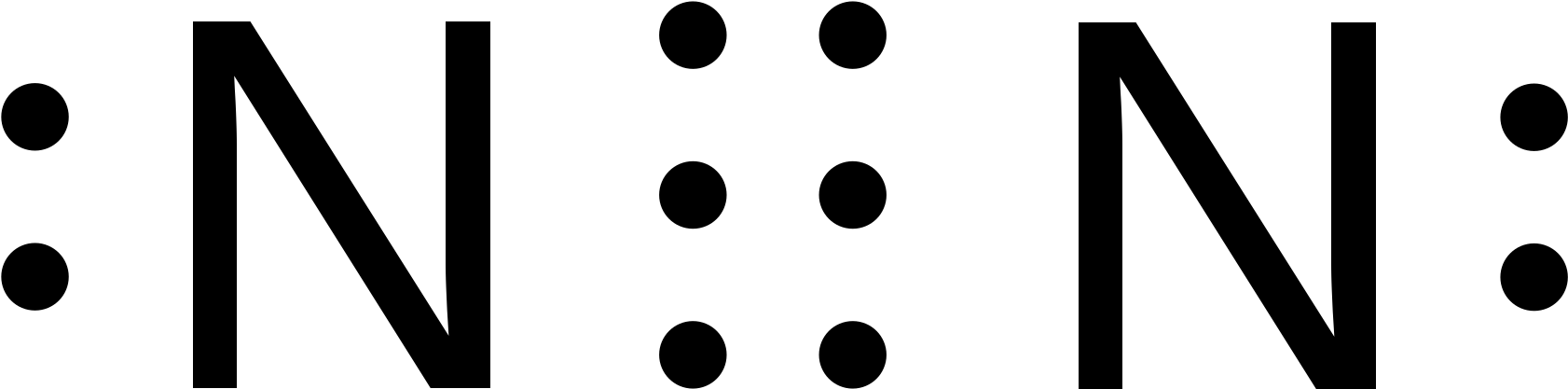
Lewis diagram for n2. A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t... In the lewis structure of N2, there is a triple bond between two nitrogen atoms. The molecular geometry of N 2 is linear. N2 is colorless, odorless, and tasteless gas. Each nitrogen atom is surrounded by a lone pair of electrons. There are three half-filled 2p orbitals in the valence shell of the nitrogen atom. Dec 25, 2017 · The input is from a 4 KW, 1400 rpm motor. Draw the speed diagram and indicate the number of teeth on each gear in a kinematic diagram. [AU, May / Jun – 2007] 4.75) Design a 9 speed gear box to give output speeds between 280 and 1800rpm. The input power is 5.5kW at 1400rpm. Draw the kinematic layout diagram and the speed diagram. The N 2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair.
The two letter N's in the N2 Lewis structure represent the nuclei (centers) of the nitrogen atoms. Furthermore, Which is the correct Lewis structure for Carbononitridic?, The Lewis structure should be this ⇒ Cl-C≡N, accompanied by 3 lone pairs on the Cl and one lone pair on the N. lewis structure n2 Nitrogen is a triple bonded molecule. Since Nitrogen belongs to the diatomic molecule in the VA family, on the periodic tables, which means that the valency of the molecule is five, therefore, it needs three more valences of electrons in order to complete its octet, and therefore, it is a triple bonded molecule. Answer: The Lewis structure for Nitrogen is as follows You can see the triple bond with the lone pair on both of the nitrogen atoms. The molecular orbital diagram for nitrogen is as follows You can see the accounting for each of the valence electrons (5 from each atom) place them into three bo... Academia.edu is a platform for academics to share research papers.
Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2. Click here👆to get an answer to your question ️ Which of the following represent the lewis structure of N2 molecule? Solve Study Textbooks. Join / Login >> Class 11 >> Chemistry >> Chemical Bonding and Molecular Structure ... The Lewis dot diagram representing ammonia molecule is: Marshall County is a county located on the south central border of Oklahoma. As of the 2010 census, the population was 15,840.[1] Its county seat is Madill.[2] Drawing the Lewis Structure for N 2 Viewing Notes: Make sure you count the number of valence electrons correctly. Nitrogen is in group 5A (also called Group 15). Each Nitrogen atom has five valence electrons. Since there are two Nitrogen atoms in N 2 you have a total of ten valence electrons to work with.
Drawing the Lewis Structure for N 2 (Dinitogen or Nitrogen Gas). Nitrogen (N 2) is a commonly tested Lewis structure due to its importance on Earth (about 78% of the Earth's atomsphere is N 2).It also is a good example of a molecule with a triple bond. There are 10 valence electrons available for the Lewis structure for N 2.. Video: Drawing the Lewis Structure for N 2
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. Here is the electron dot structure for a single N atom: The total number of valence electrons between the two N atoms is 10 e^-.
How to Draw the Lewis Structure of N2 - with explanation!Check me out: http://www.chemistnate.com
Considering the energy level diagram, the configuration of N2 is σ1S2, σ *1S2, σ2S2, σ*2S2, π2Px2, π2Py2, σ2Pz1. Conclusion In the Lewis structure of the N2 molecule, there is a formation of a triple covalent bond represented by three lines between two atoms of Nitrogen.
Answer (1 of 2): In exactly the same way as you'd do it for any simple, covalently bonded compound. First you need to know the valency of each element. Then you need to draw a stick model with bonds between the atoms, so that each atom's valency is satisfied (so N has three bonds coming out of i...
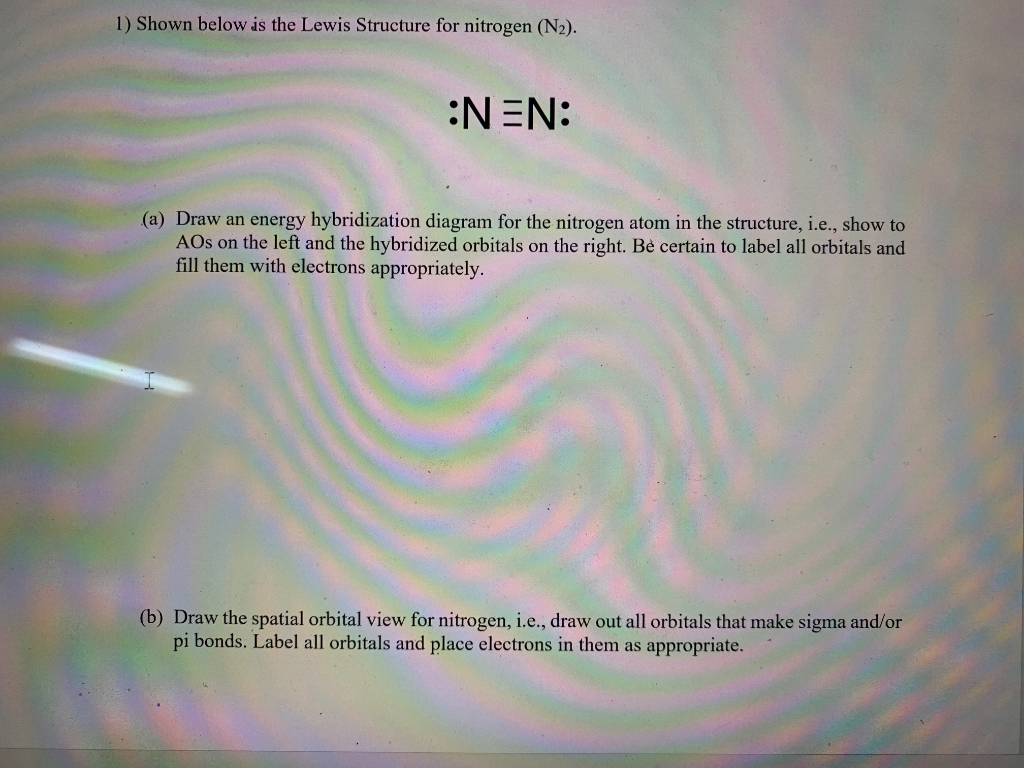
Transcribed image text: (1) How does the Lewis structure of N2 compare with its molecular orbital diagram, in terms of bond order and the number of unpaired electrons? (1.e., state the bond order and number of unpaired electrons indicated by the Lewis structure, then state the bond order and number of unpaired electrons indicated by the MO diagram.)
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (8 ratings) Th …. View the full answer. Transcribed image text: Draw the Lewis structure of N2. Previous question Next question.
31 Dec 2015 — In N2 Lewis structure,two nitrogen atoms has shared six valence electrons and every nitrogen atom has one lone pairs .In N2 Lewis structure,there are ten ...3 answers · 4 votes: As nitrogen is in fifth group in periodic table therefore it will have five electrons in the ...How can you determine the Lewis dot diagram for N2? - Quora9 May 2017How does the Lewis structure of N2 related to the molecular ...30 Apr 2017What is the total number of bonding electrons in N2? - Quora1 Jan 2019What is the Lewis Dot Structure for Mg3N2? - Quora22 Oct 2018More results from www.quora.com
Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure. N 2 lewis structure There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. There are several interesting steps in drawing nitrogen's lewis structure.
Nitrogen sinks in some knee structure or nearby structure could be physical, chemical, or physiological. The authors conclude that these unexpected results of a very marked delay in knee gas excretion 30 minutes into the pulmonary washout period suggests that a gas exchange model consistent with these data is needed to avoid decompression sickness.
What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable.
The N 2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair.
Expression during development. During development, one of the major lineages expressing Pax3 is the skeletal muscle lineage. Pax3 expression is first seen in the pre-somitic paraxial mesoderm, and then ultimately becomes restricted to the dermomyotome, which forms from the dorsal region of the somites.
N2 Lewis Structure Setup It's easiest to think in terms of dots to make the N 2 Lewis structure. Nitrogen needs to bond three times, shown as the lone dots on the left, right and bottom of the N atoms in the below diagram. There is also a pair of dots, representing two more electrons, that won't bond, on top of each N.
Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure.
1. Complete the Lewis dot structure(s) for 2. Complete the Lewis dot sttllcture(s for 3. Complete the Lewis dot structure(s) for COC12 4. Complete the Lewis dot structure(s) for NOY :8-N-ë 5, Complete the Lewis dot structure(s) for C103 STOP Your group will check your answers with the instructor before moving on. Model VI: Multi-Centered Molecules





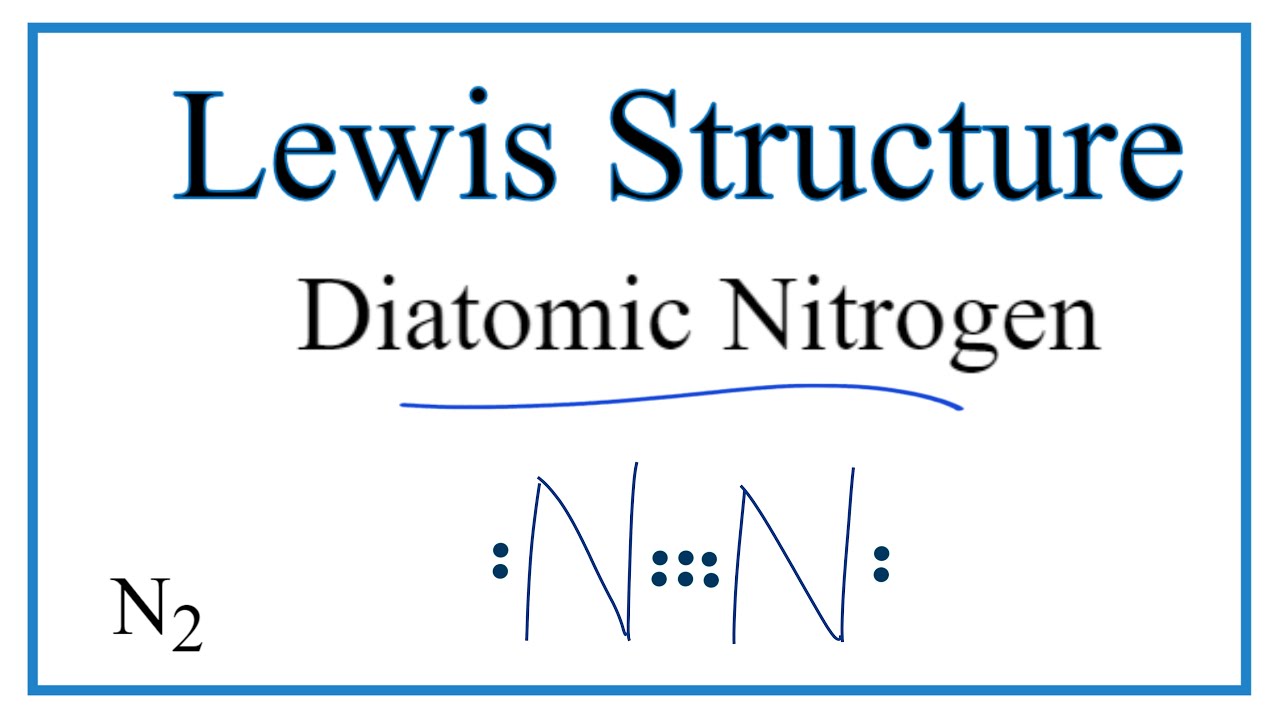
![Expert Answer] electron dot structure of Nitrogen molecule ...](https://hi-static.z-dn.net/files/d68/c596d1dd5842287e6938ce11c21acb41.png)


















0 Response to "36 lewis diagram for n2"
Post a Comment