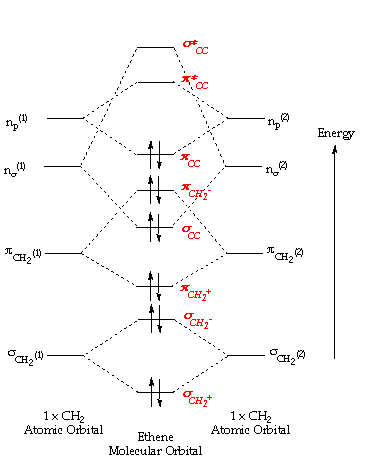
35 Ethylene Molecular Orbital Diagram
Test Bank of Organic Chemistry 4th Edition By Janice Smith ... A) sp3 B) sp2 C) sp D) p 52. Which atomic orbitals overlap to form the C-H bonding molecular orbitals of ethane, CH3CH3? A) Csp2 + H1s B) Csp3 + H1s C) C2p + H1s D) Csp + H1s 53. Which atomic orbitals overlap to form the C-H bonding molecular orbitals of ethylene, H2C=CH2? A) C2p + H1s B) Csp + H1s C) Csp3 + H1s D) Csp2 + H1s 54. Difference Between Bonding And Antibonding Orbitals ... This combines s orbital with one p orbital. This means that s and p are equal. Sp 2 example of is ethylene. This is combination of one s orbital and two p orbitals. Sp 3 of this is methane. This is combination of one s orbital and three p orbitals. If you add exponents of hybridized orbitals, you get amount of sigma bonds associated with that bond.
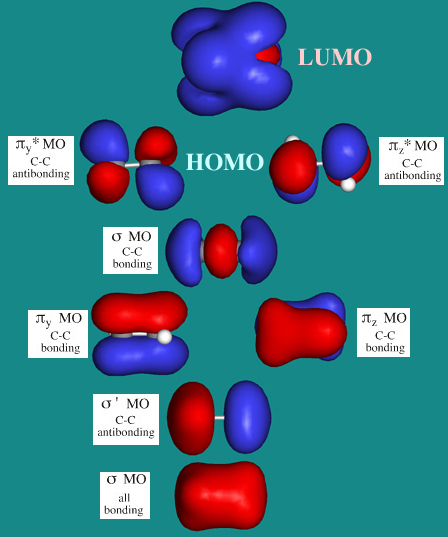
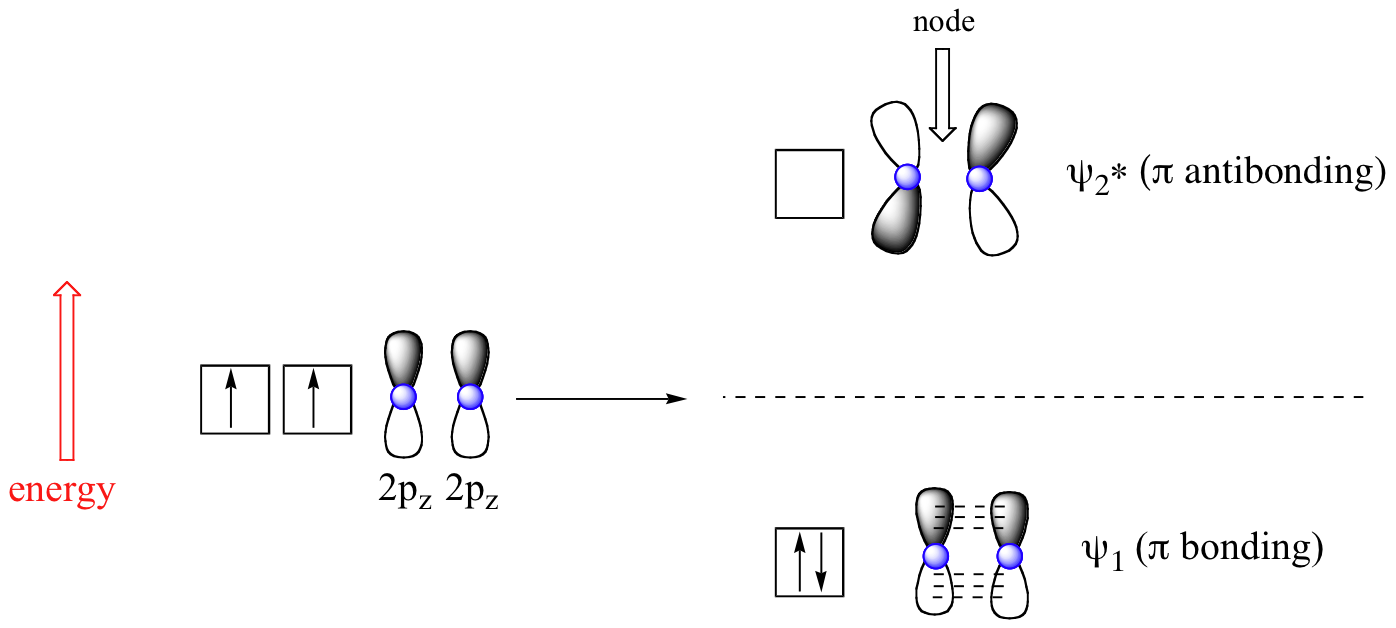
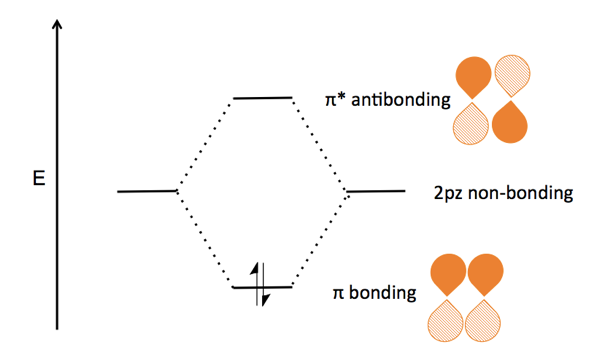
› pi-molecular-orbitalsIntroduction to Pi Molecular Orbitals Ethylene - Chad's Prep® Pi Molecular Orbitals of Ethylene. In ethylene there are two adjacent carbon atoms involved in the pi system and the combination of a p orbital from each of these atoms will result in two pi molecular orbitals: ψ1 and ψ2*, (also referred to as π1 and π2*). ψ1 is a bonding molecular orbital, is occupied in the ground state, and is the ...

Ethylene molecular orbital diagram
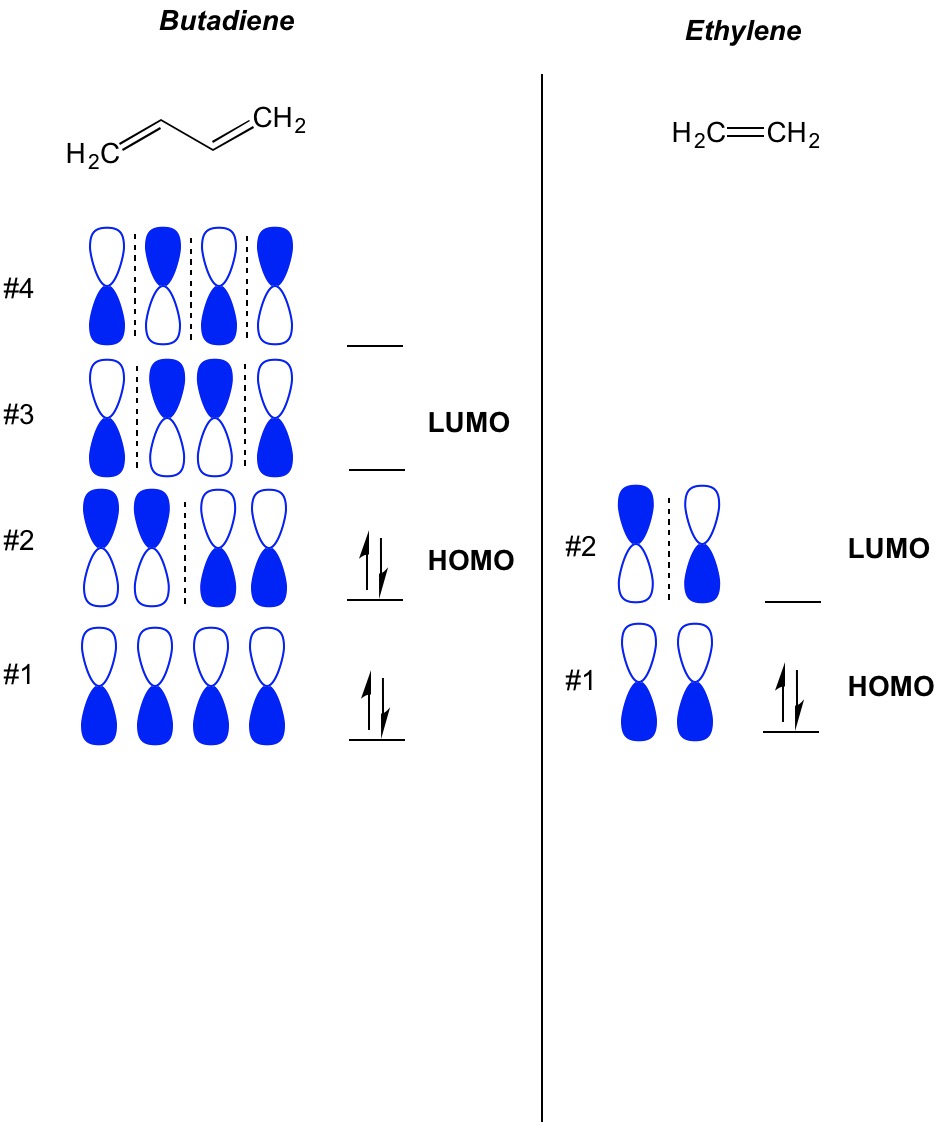
schematron.org › ethene-molecular-orbital-diagramEthene Molecular Orbital Diagram - schematron.org Oct 22, 2018 · The molecular orbital diagram for the π-molecular orbitals of butadiene as a result of combining the π-molecular orbitals of two ethene molecules. This shows .Bonding orbitals in Ethene (Ethylene) sp 2 Background: Use the buttons to display the sp 2 orbitals that make up the sigma framework and the remaining p orbitals which form the pi-bond. C2H4 Lewis Structure, Molecular Structure, Hybridization ... The chemical formula C2H4 represents Ethylene. This molecule is also represented by H2C=CH2, clearly showing the alkene nature of the compound. An alkene is a hydrocarbon with a Carbon-Carbon double bond. C2H4 exists as a colorless gas and is flammable. Its odor is described as faintly 'sweet and musky'. What are lobes in chemistry? - Kitchen The π orbital of ethylene has two orbital lobes (one shown in the red and the other in blue), and one orbital node (the plane which contains the atoms). What are lobes in p orbital? Visualizing Electron Orbitals The number of possible values is the number of lobes (orbitals) there are in the s, p, d, and f subshells.
Ethylene molecular orbital diagram. lec2 - 2021-07-13T160143.414.pdf - Pericyclic Reactions ... Now, here on the left-hand side, the ethylene pi molecular orbitals are given. Ethylene has two pi electrons. That means, it should have two molecular orbitals. The pi molecular orbital, and the pi-star ( *) molecular orbital. This is the occupied molecular orbital and, this is an empty molecular orbital. Ethane Formula: C₂H₆ Structure, Preparation, Uses - Embibe Out of these eight orbitals, six orbitals are involved in sigma bonding with the hydrogen atoms. The remaining two hybridised orbitals overlap with each other to form a sigma bond. This is diagrammatically represented as below- Molecular Geometry of Ethane Ethane can be viewed as a dimer of a methyl group. C2H6 lewis structure: Etane Hybridization, Molecular ... C2H6 lewis structure: Ethane Hybridization, Molecular Geometry and shape. Ethane is an organic compound with a chemical formula of C2H6. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. RILQWHUIHUHQFHHIIHFWVLQ H Characterization of Electronic ... molecular targets and MOs studied are still scarce and further investigations are required to develop and enhance the potential ability of bond oscillation. Under the above-mentioned circumstances, we have carried out EMS measurements for the outermost orbitals of CO 2, ethylene and 1,3-butadiene, i.e. the 1π g,1b 3u and 1b g orbitals ...
Samacheer Kalvi 11th Chemistry Guide Chapter 10 Chemical ... Molecular orbital diagram of Carbon monoxide molecule (CO) Electronic configuration of C atom: 1s 2 2s 2 2p 2 ... The molecular formula of ethylene is C 2 H 4. The valency of carbon is 4. The electronic configuration of valence shell of carbon in ground state is [He] 2s 2 2p x 1 2p y 1 2p z 0. What are lobes in chemistry? - Vintage Kitchen The π orbital of ethylene has two orbital lobes (one in red and the other in blue) and an orbital node (the plane that contains the atoms). Innehåll dölja. ... The total number of molecular orbitals equals the total number of atomic orbitals used to make them. Molecular Orbital Theory Multiple Choice Questions Pdf VSEPR theory (molecular shapes), bond polarity, molecular polarity and IMFs ... 213XZ948 Felicia Mo AP Chemistry Unit 2,3 Question #1 1a) 1b) C2H2 ... There are 60 multiple-choice questions and 7 free- response questions.. orbitals (called molecular orbitals) spread over entire molecule. diagramweb.net › molecular-orbital-diagram-ofMolecular Orbital Diagram Of Ethene Ethene This sideways overlap also creates a molecular orbital, but of a different kind. In this. Ethylene is the simplest molecule that has a double bond. As we saw from the valence bond model, we should find the presence of a σ-bond framework, and a . Bonding orbitals in Ethene (Ethylene) sp2. sp2 hybrids. ethylene orbitals.
CHEM101: The Hybrid Orbital Model | Saylor Academy These three views of the ethylene molecule emphasize different aspects of the disposition of shared electron pairs in the various bonding orbitals of ethene (ethylene). (a) The backbone structure consisting of σ ( sigma ) bonds formed from the three sp 2 -hybridized orbitals on each carbon. Ethene (ethylene): Molecular Geometry - Hybridization ... The hybrid orbitals are more prominent outward so that their ability to overlap is stronger than that of normal orbitals. Molecular Formula: A chemical formula is a brief way of expressing the number and type of atoms that make up a particular chemical compound. walsh diagram for tri and penta atomic molecules pdf 98 By plotting the change in molecular orbital levels of a molecule as a function of ... a correlation diagram for the possible orbitals of a polyatomic molecule in two ... pentaatomic molecules (CH3I), hexaatomic molecules (ethylene), and .... atoms of a diatomic molecule a and b, so the atomic orbital wave ... orbital diagrams in this book (such ... Quick Answer: How Many Electrons Are Shared Between The ... In the ethylene molecule, each carbon atom is bonded to two hydrogen atoms. The unhybridized pz orbitals on each carbon overlap to a π bond (pi). The orbital overlap is commonly written as p z (C)-1p z (C). How many carbon atoms does C2H2? The chemical formula for acetylene is C2H2 and the chemical formula for carbon monoxide is CO.
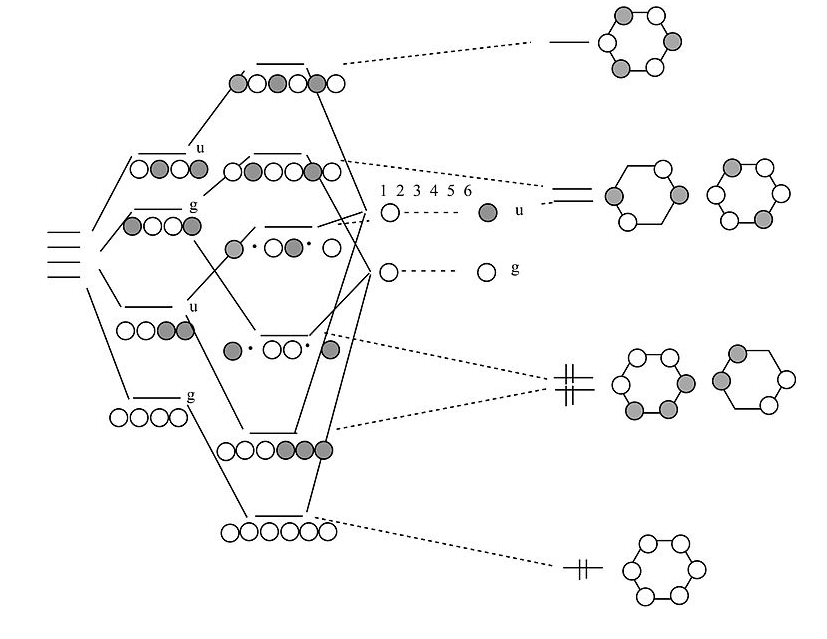
construct mo diagram of 1 3 butadiene universal bench grinder stand; mental health center inpatient; how to send money from western union to gcash; giant schnoodle vs goldendoodle; roger and talia scott net worth
How to calculate the energy levels for ... - ResearchGate This can be readily obtained if you represent the molecular orbital of acetylene as a linear combination of four 2p- atomic orbitals (from two different centers) and solve the stationary ...
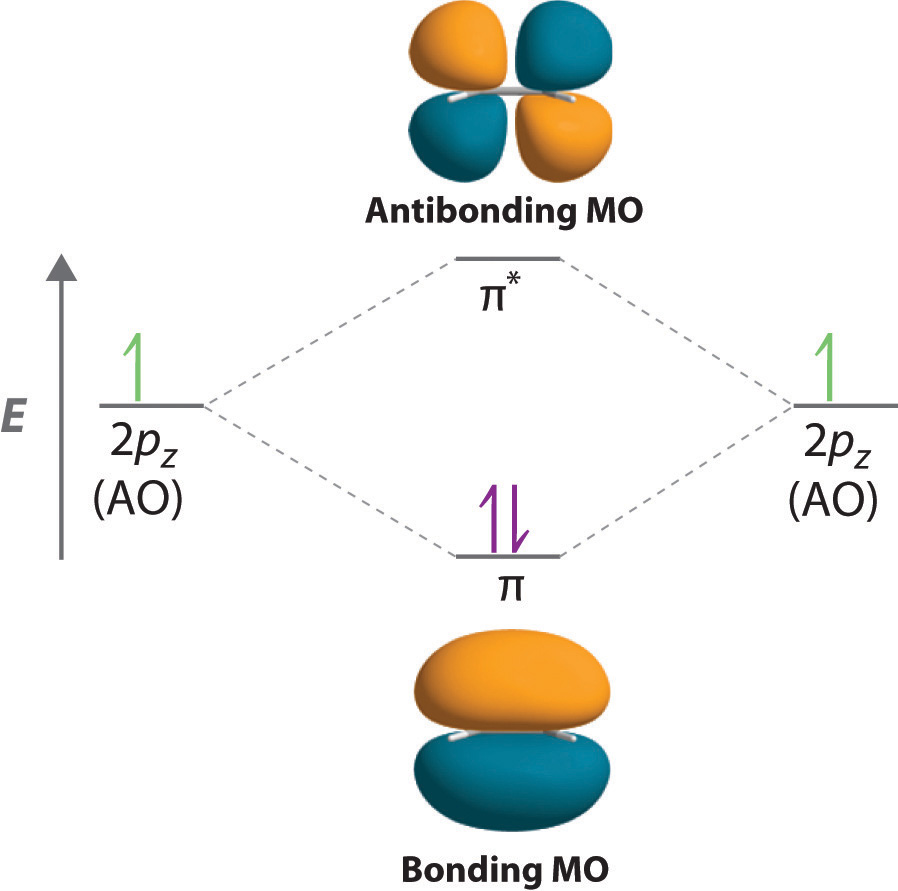
1.2.5: Multiple Covalent Bonds - Chemistry LibreTexts Figure 1.2.5. 2: Molecular Orbital Energy-Level Diagram for π Bonding in Ethylene. As in the diatomic molecules discussed previously, the singly occupied 2 pz orbitals in ethylene can overlap to form a bonding/antibonding pair of π molecular orbitals. The two electrons remaining are enough to fill only the bonding π orbital.
1.8: sp² Hybrid Orbitals and the Structure of Ethylene ... An ethylene molecule is said to be made up of five sigma bonds and one pi bond. The three sp 2 hybrid orbitals on each carbon orient to create the basic trigonal planer geometry. The H-C-C bond angle in ethylene is 121.3 o which is very close to the 120 o predicted by VSEPR. The four C-H sigma bonds in ethylene .
ursula.chem.yale.edu › MO-HTMLs › ethylene-log-3π- Molecular Orbitals (MO's) of Ethylene - Yale University 14 molecular orbitals read in model 2. 0 molecular orbitals read. Mulliken charges found for Model 2. Molecular dipole for model 2 = {0, 0, 0} Time for openFile (./mo/ethylene.log): 101 ms. reading 12 atoms. ModelSet: haveSymmetry:false haveUnitcells:false haveFractionalCoord:false. 2 models in this collection.
what orbitals are used to form the indicated bond ... In the ethylene molecule, each carbon atom is bonded to two hydrogen atoms. Thus, overlap two sp2-hybridized orbitals with the 1s orbitals of two hydrogen atoms for the C-H sigma bonds in ethylene (sp2(C)-1s (H). How do you identify orbitals? Each orbital is denoted by a number and a letter.
difference between sigma and pi molecular orbitals difference between sigma and pi molecular orbitals. by | Feb 3, 2022 | what is branch and home office accounting | Feb 3, 2022 | what is branch and home office accounting
How To Draw Molecular Orbital Diagram Of Co - Drawing ... Molecular orbital diagram for oxygen gas (o2).fill from the bottom up, with 12 electrons total.bonding order is 2, and it is paramagnetic.sigma2s(2),sigma2s*. Building molecular orbital diagrams for homonuclear and heteronuclear diatomic molecules; (1) e n = 13.6 z e f f 2 n 2 e v.
What are lobes in chemistry? - Vintage Kitchen The π orbital of ethylene has two orbital lobes (one in red and the other in blue) and an orbital node (the plane that contains the atoms). ... 12 How many electrons can a molecular orbital hold? ... Electron Orbital Display The number of possible values is the number of lobes (orbitals) found in the s, p, d, and f subshells. As shown in Table ...
Quick Answer: How many molecular orbitals Does benzene ... Pi orbital (π orbital): The bonding molecular orbital component of a pi bond. The π orbital of ethylene's carbon-carbon pi bond has two orbital lobes, one above the plane of the atoms, and another below the plane. This is a bonding molecular orbital. The plane containing the atoms is also the pi orbital's one node.
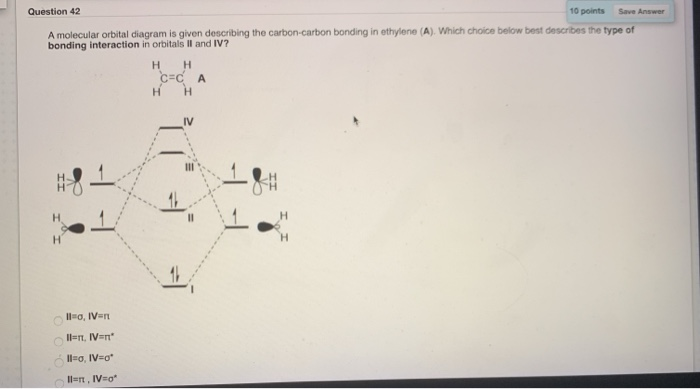
› ~vederas › Chem_164Molecular Orbitals: Example 1: Ethylene - ualberta.ca Molecular Orbitals: Example 1: Ethylene E E H2CCH2 p 2 sp2 1s p 2 sp2 1s σ σ* π π* A.O. A.O. C C HHC C C from other end of double bond A.O. means atomic orbitals (s, sp2, p) M.O. means molecular orbitals (σ , π ) C from left end of double bond M.O. Looking at both sigma and pi bonds
sites.science.oregonstate.edu › ethylene_MOsMolecular Orbitals: Ethene (Ethylene) Apr 14, 2014 · Molecular Orbitals: Ethene (Ethylene) Ethylene MOs. Ethylene is the simplest molecule that has a double bond. As we saw from the valence bond model, we should find the presence of a σ-bond framework, and a π-bond between carbons. Jmol._Canvas2D (Jmol) "Ethylene" [x]
Ethylene Molecule - media portfolio, c2h4 lewis structure ... c2h4 lewis structure molecular geometry hybridization. Ethylene Molecule. Here are a number of highest rated Ethylene Molecule pictures upon internet. We identified it from well-behaved source. Its submitted by organization in the best field. We acknowledge this kind of Ethylene Molecule graphic could possibly be the most trending subject ...
What are lobes in chemistry? - Kitchen The π orbital of ethylene has two orbital lobes (one shown in the red and the other in blue), and one orbital node (the plane which contains the atoms). What are lobes in p orbital? Visualizing Electron Orbitals The number of possible values is the number of lobes (orbitals) there are in the s, p, d, and f subshells.
C2H4 Lewis Structure, Molecular Structure, Hybridization ... The chemical formula C2H4 represents Ethylene. This molecule is also represented by H2C=CH2, clearly showing the alkene nature of the compound. An alkene is a hydrocarbon with a Carbon-Carbon double bond. C2H4 exists as a colorless gas and is flammable. Its odor is described as faintly 'sweet and musky'.
schematron.org › ethene-molecular-orbital-diagramEthene Molecular Orbital Diagram - schematron.org Oct 22, 2018 · The molecular orbital diagram for the π-molecular orbitals of butadiene as a result of combining the π-molecular orbitals of two ethene molecules. This shows .Bonding orbitals in Ethene (Ethylene) sp 2 Background: Use the buttons to display the sp 2 orbitals that make up the sigma framework and the remaining p orbitals which form the pi-bond.










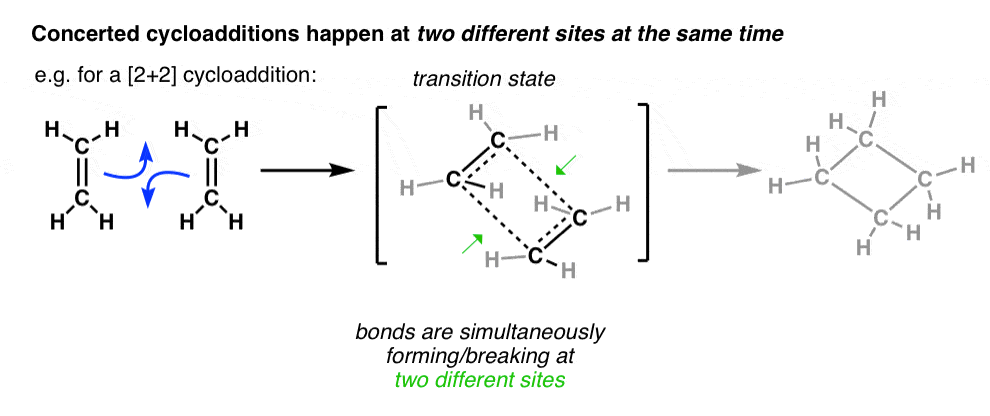

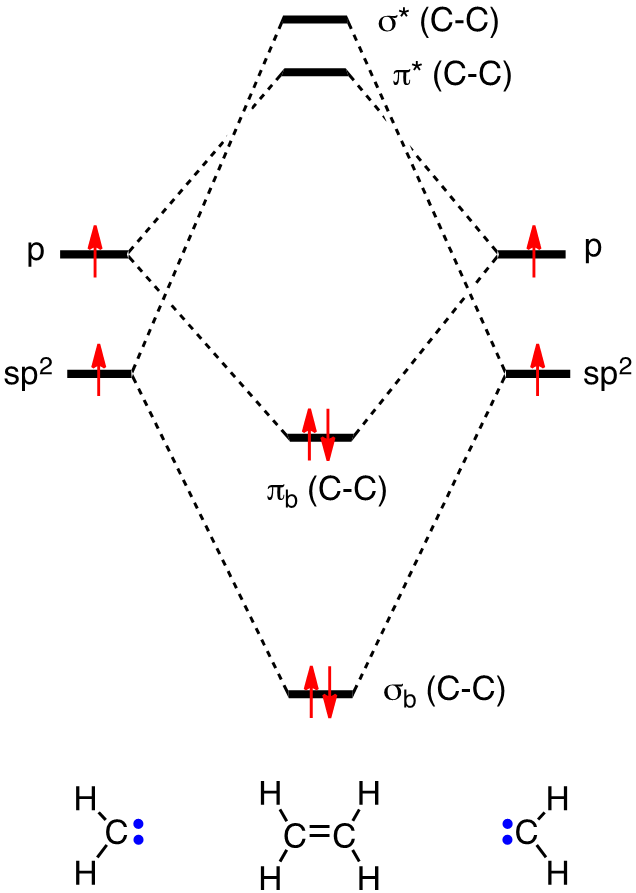




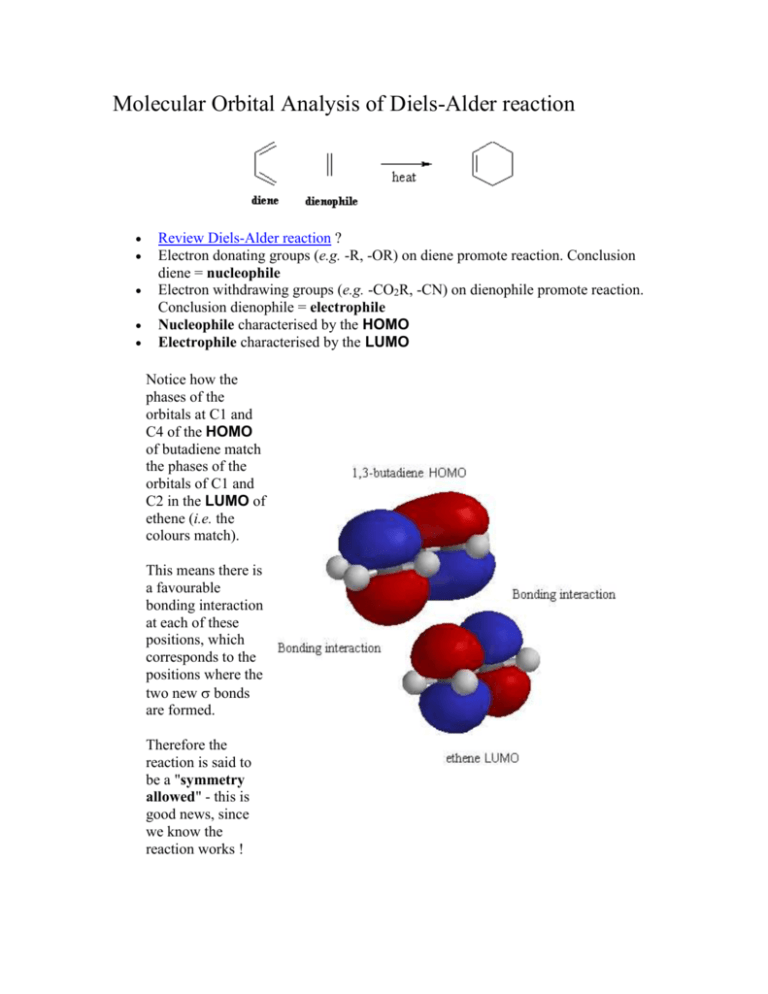
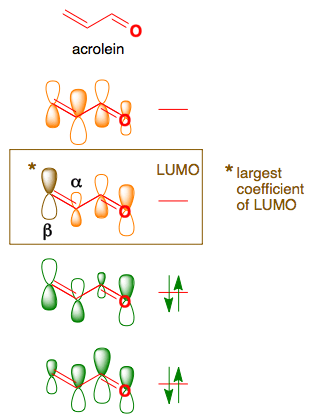

0 Response to "35 Ethylene Molecular Orbital Diagram"
Post a Comment