36 Xef Molecular Orbital Diagram
Molecular Orbital Diagram Maker ©2022 Prof Adam J Bridgeman | close window : ©2022 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window PDF Chem 130 - Second Exam Key - DePauw University the molecular orbitals. The complete molecular orbital diagram shows that (a) sulfur is on the left and oxygen is on the right, that (b and c) sulfur's valence shell is 3s23p4 and oxygen's valence shell is 2s22p4, and that (d) the 12 total valence electrons fill the molecular orbitals from the bottom-to-top, filling each
XeF2 bonding - YouTube In this screencast, Andrew Burrows uses molecular orbital theory to explain the bonding in xenon difluoride. ...

Xef molecular orbital diagram
PDF Lecture 4 d orbitals - University of Oxford d orbitals improve bonding, but we can explain the stability of XeF 2 without them They do not participate to the extent implied by a formal sp 3d hybridisation model unrealistic more realistic. SF 6 sp 3d2 hybrids? ionic resonance? S-F bond order = 4/6. Solved Although KrF+ and XeF+ have been studied, KrBr+ has ... Although KrF + and XeF + have been studied, KrBr + has not yet been prepared. For KrBr +:. a)Propose a molecular orbital diagram showing the interactions of the valence shell s and p orbitals to form molecular oribtals Hybridization of XeF2 - Hybridization of Xe in Xenon ... There are three hybrid orbitals that contain the lone pairs and they do not form any bonds. XeF2 Molecular Geometry And Bond Angles. XeF2 molecular geometry is linear. It acquires such shape as the lone pairs present around the central atom tend to take up equatorial positions. The bond angle is said to be 180°.
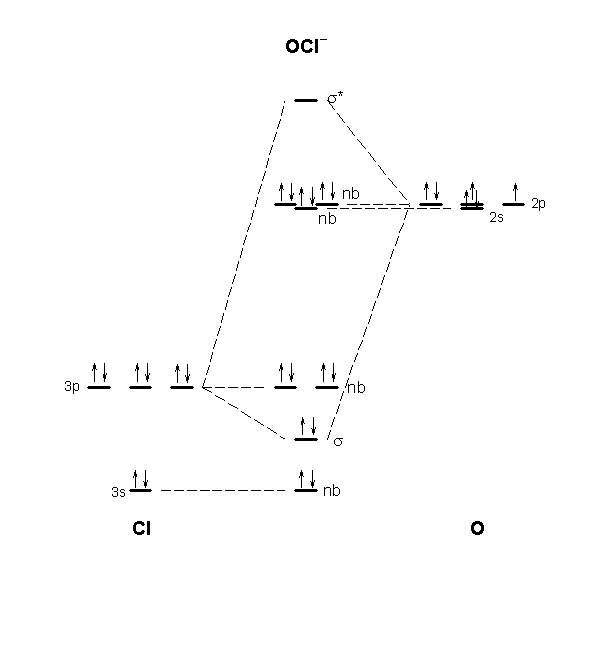
Xef molecular orbital diagram. Hybridization of XeF4 - Explanation, Structure and ... These orbitals transfer to complete the empty 5d orbitals in the process of making the XeF 4. This results in 4 unpaired hybridized electrons which consist of 2 in 5p and 2 in 5d orbitals. So, finally, we get the actual orbital used in XeF 4 development, and it results in sp 2 d 2 hybridization. Answered: Sketch the molecular orbital energy… | bartleby Answered: Sketch the molecular orbital energy… | bartleby. Hit Return to see all results. Science. Chemistry Q&A Library Sketch the molecular orbital energy level diagram for XeF and deduce its ground-state electron configuration. Is XeF likely to have a shorter bond length than XeF+? What is the molecular geometry of XeF3? - FindAnyAnswer.com What is the molecular geometry of XeF3? Secondly, because all three of the lone pairs are structurally significant, they contribute to the electron-pair geometry. Therefore 3 F atoms + 3 lone pairs = 6 bonded structures. Therefore, the electron-pair geometry is octahedral (here, octa = six). Molecular Structure Practice Problems Answers The molecular orbital diagram for ClO - is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated.
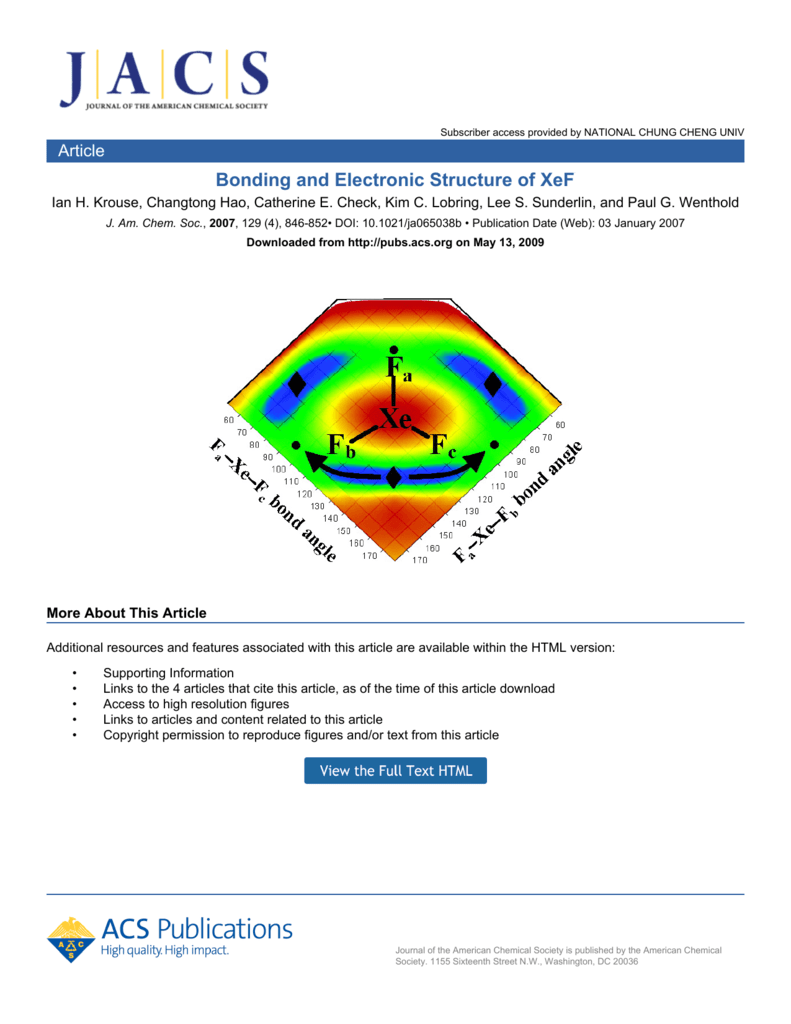
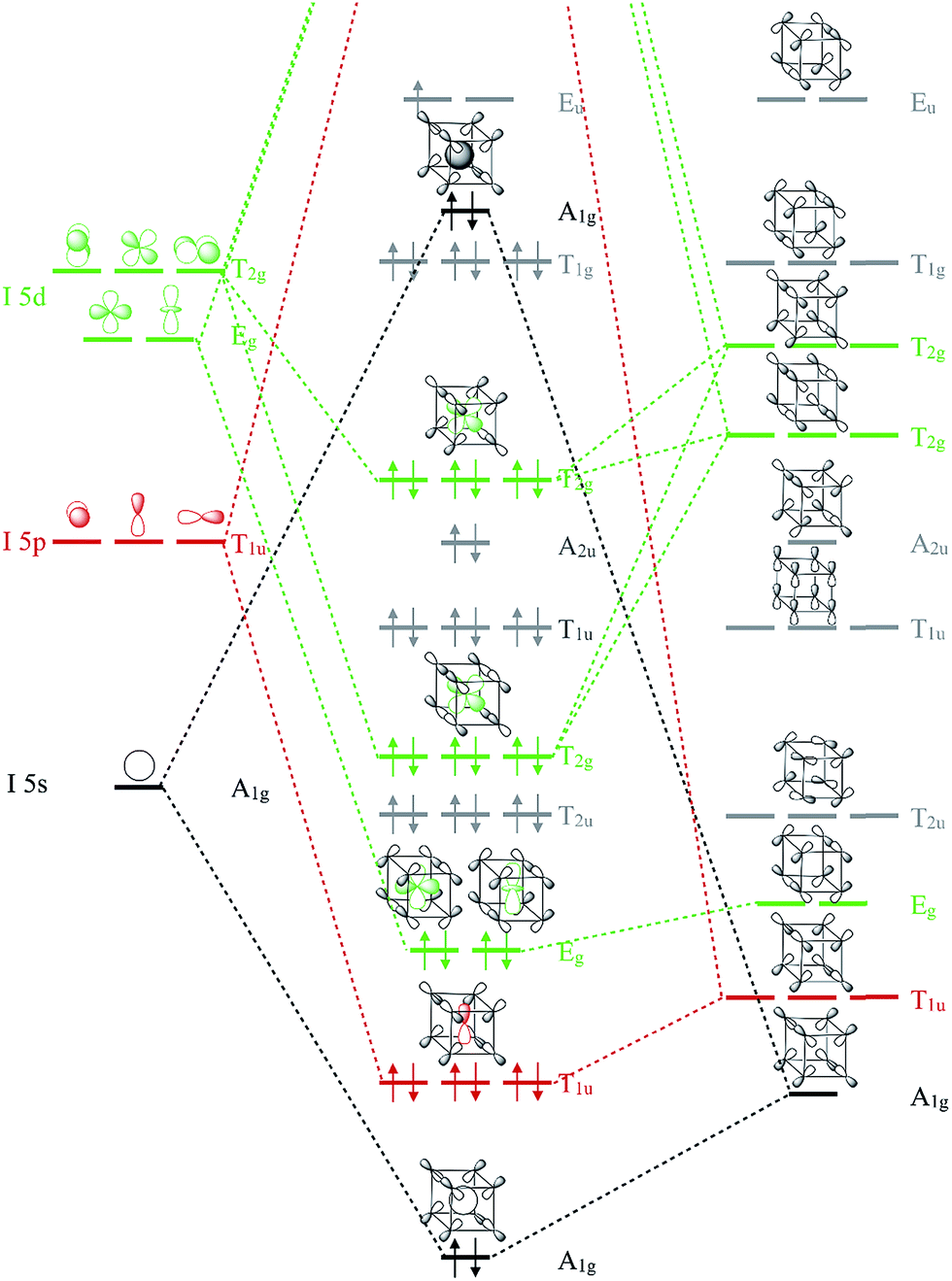
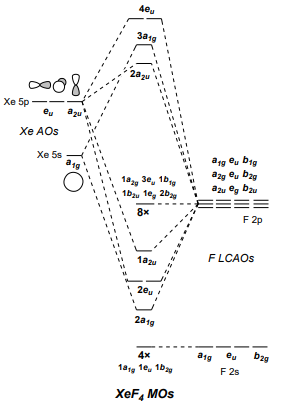
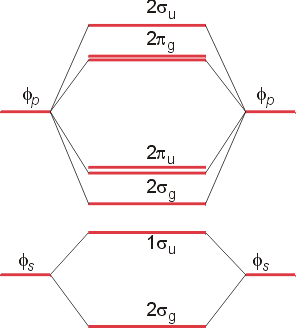
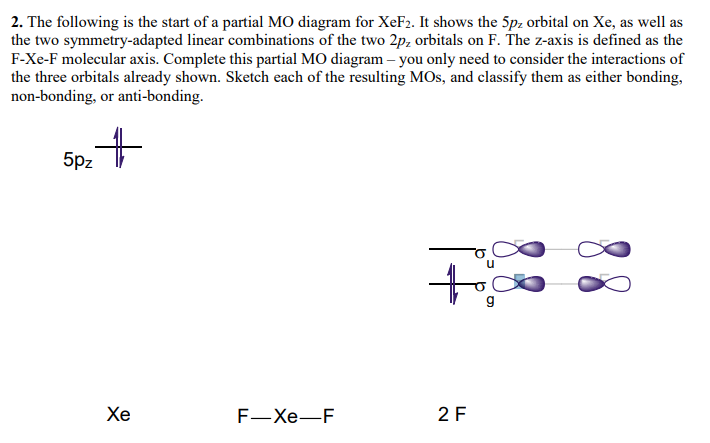
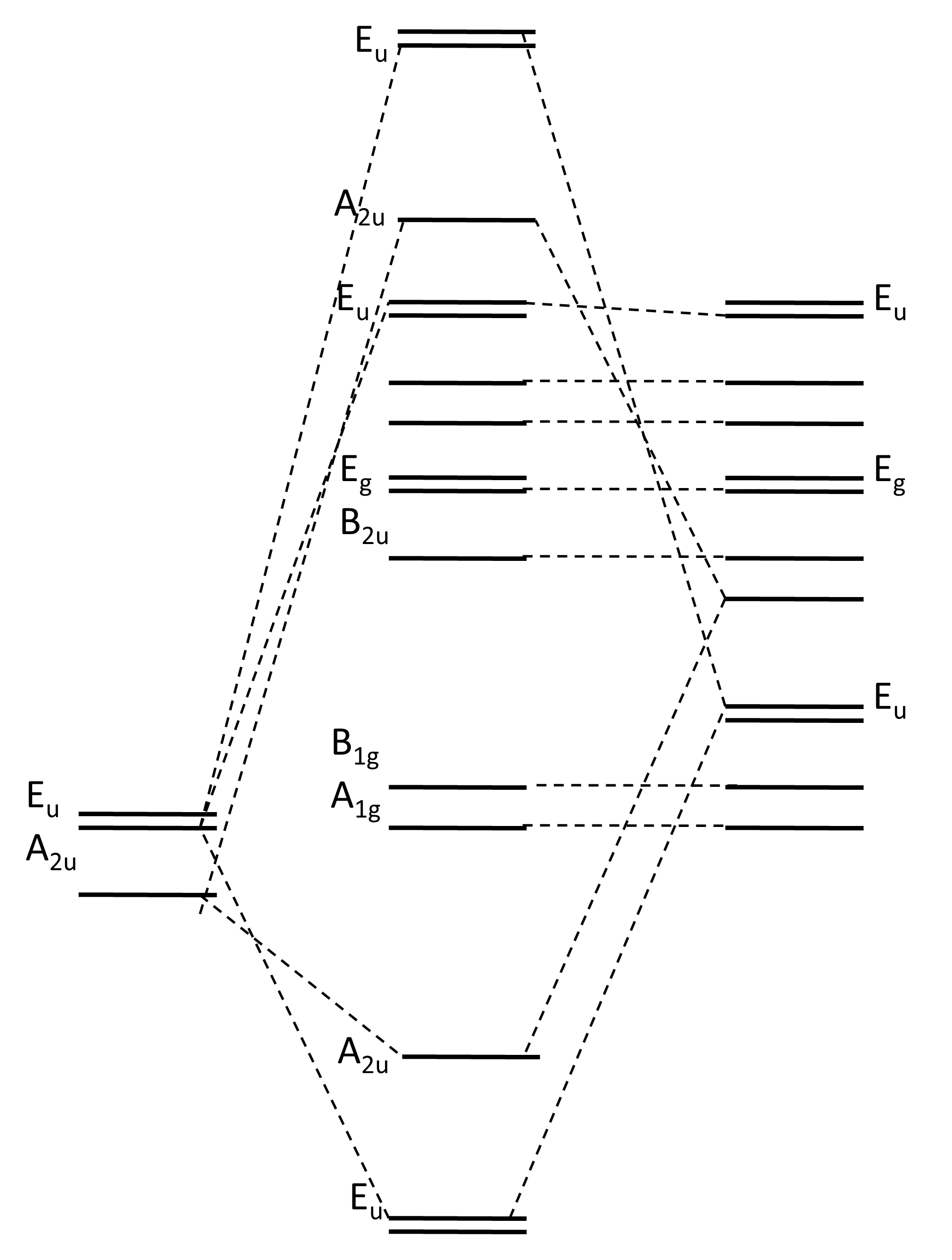
XeF4 Lewis Structure, Molecular Geometry, Hybridization ... MO Diagram of XeF4 An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of molecular orbital theory. When atoms combine with other atoms to make molecules, some of the atomic orbitals adds up to form molecular orbitals which are the same in number. (PDF) Bonding and Electronic Structure of XeF 3 | Kim ... Download. Bonding and Electronic Structure of XeF 3. Kim Lobring. Subscriber access provided by NATIONAL CHUNG CHENG UNIV Article Bonding and Electronic Structure of XeF 3- Ian H. Krouse, Changtong Hao, Catherine E. Check, Kim C. Lobring, Lee S. Sunderlin, and Paul G. Wenthold J. Am. Chem. Soc., 2007, 129 (4), 846-852• DOI: 10.1021/ja065038b ... inorganic chemistry - Chemistry Stack Exchange How to get those correlations, is quite a fair question. Well, I got it from this diagram in Orbital Interactions in Chemistry, 2nd ed. by Albright et al.The correlation diagram 14.4 should be self-explanatory, hopefully (you can ignore the pictures of the MOs, 14.5 and 14.6).. But if you want to obtain it without flipping through every book in the library, this is how you need to do it. Solved Create a molecular orbital diagram for XeF4 | Chegg.com Create a molecular orbital diagram for XeF4 assuming the F atoms only participate in sigma bonding with the center. Indicate corresponding symmetry. (For Xe, E5s=-23.4 eV, E5p= -12.6 eV, E5d= -3 eV; for F E2s= -40.2 eV and E2p= -18.7 eV)
Hybridization of XeF6 - Hybridization of the Xenon atom in ... XeF 6 has seven electron pairs. It consists of 6 bond pairs and one lone pair. Xenon has 8 electrons in its valance shell and it forms six bonds with the fluorine atoms. When the fluorides of xenon have formed the electrons in the valence shell of xenon get unpaired and are promoted to vacant 5d orbitals. XeF 6 Molecular Geometry And Bond Angles XeF4 Molecular Geometry - Science Education and Tutorials The XeF4 molecular geometry is a diagram that illustrates the number of valence electrons and bond electron pairs in the XeF4 molecule in a specific geometric manner. PDF Inorganic Chemistry with Doc M. - Creighton University Molecular orbital diagram for square planer XeF4 6. Using p-orbitals for σ-bonding: molecular orbital diagram for trigonal planer BF3 1. Symmetry adapted linear combinations (SALC) of bonding group atomic orbitals For molecules where the central atom is bonded to more than one other B group such as the Chemical Bonding and Molecular Structure Class 11 ... In the molecular orbital diagram for O 2 + ion, the highest occupied orbital is (a) σ MO orbital (b) π MO orbital (c) π* MO orbital (d) σ* MO orbital. Answer. C. ... In XeF 6, oxidation state and state of hybridisation of Xe and shape of the molecule are, respectively (a) + 6, ...
Xenon tetrafluoride (XeF4) - D4h Symmetry - ChemTube3D Click the Symmetry Operations above to view them in 3D. XeF 4 belongs to the D 4h Point group and contains; One C 4 rotation axis, one C 2 rotation axis (equivalent to C 42 ), Four C 2 axes perpendicular to the C 4 axis. 4σ planes of symmetry,one σ h plane. One S 4 axis.
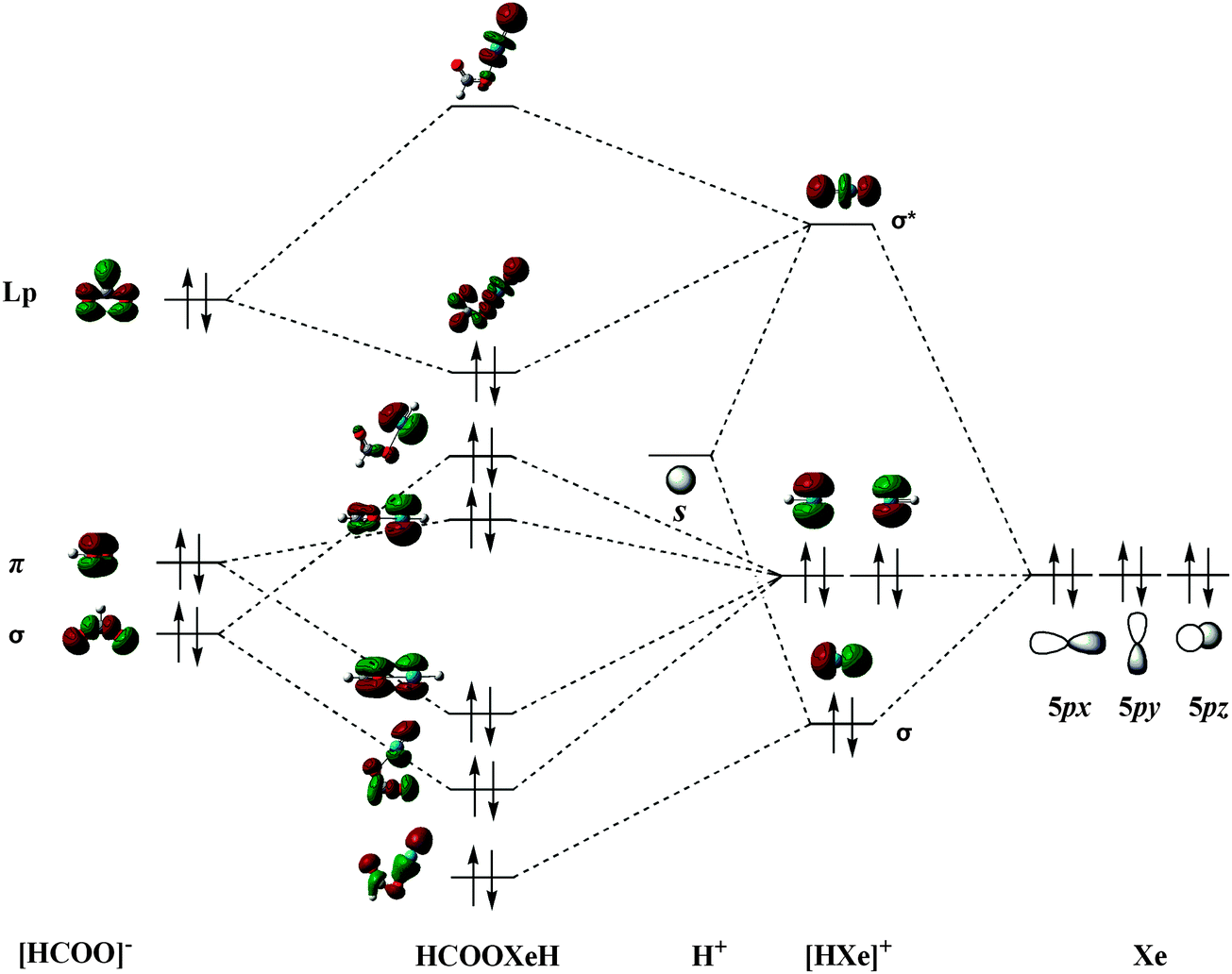
XeF2 Molecular Orbital - Australian National University The electronic configuration of XeF 2 in the ground electronic state is given in the following: (8σ g ) 2 (5σ u ) 2 (9σ g ) 2 (6σ u ) 2 (4π u ) 4 (3π g ) 4 (10σ g ) 2 (5π u ) 4 (7σ u ) 0 . The lowest vacant orbital, 7σ u , which has an antibonding character consists mainly of 5p z atomic orbitals (AO's) in Xe with a small contribution ...
molecule is XeF 4 Hybridization of Xe atom is sp 3 d 2 ... When atomic orbitals are combined to give molecular orbitals, the number of molecular orbitals formed equals the number of atomic orbitals used. A molecular orbital (like an atomic orbital) can contain no more than two electrons (Pauli Exclusion Principle), and are filled starting with the lowest energy orbital first.
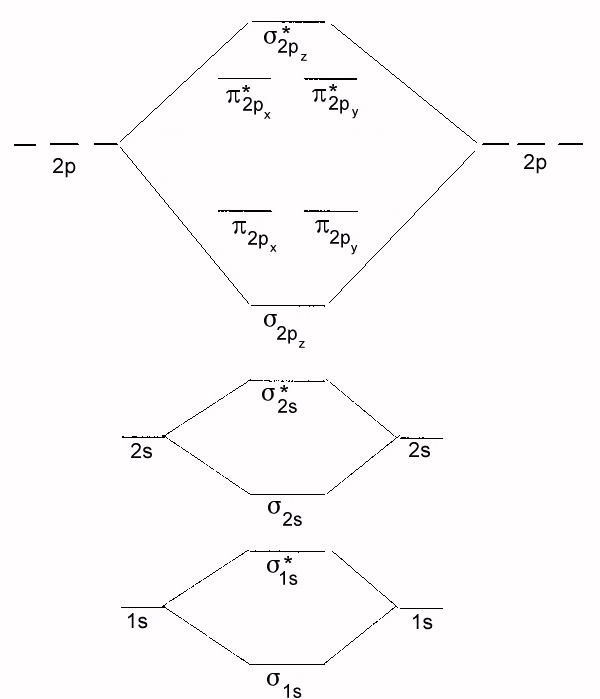
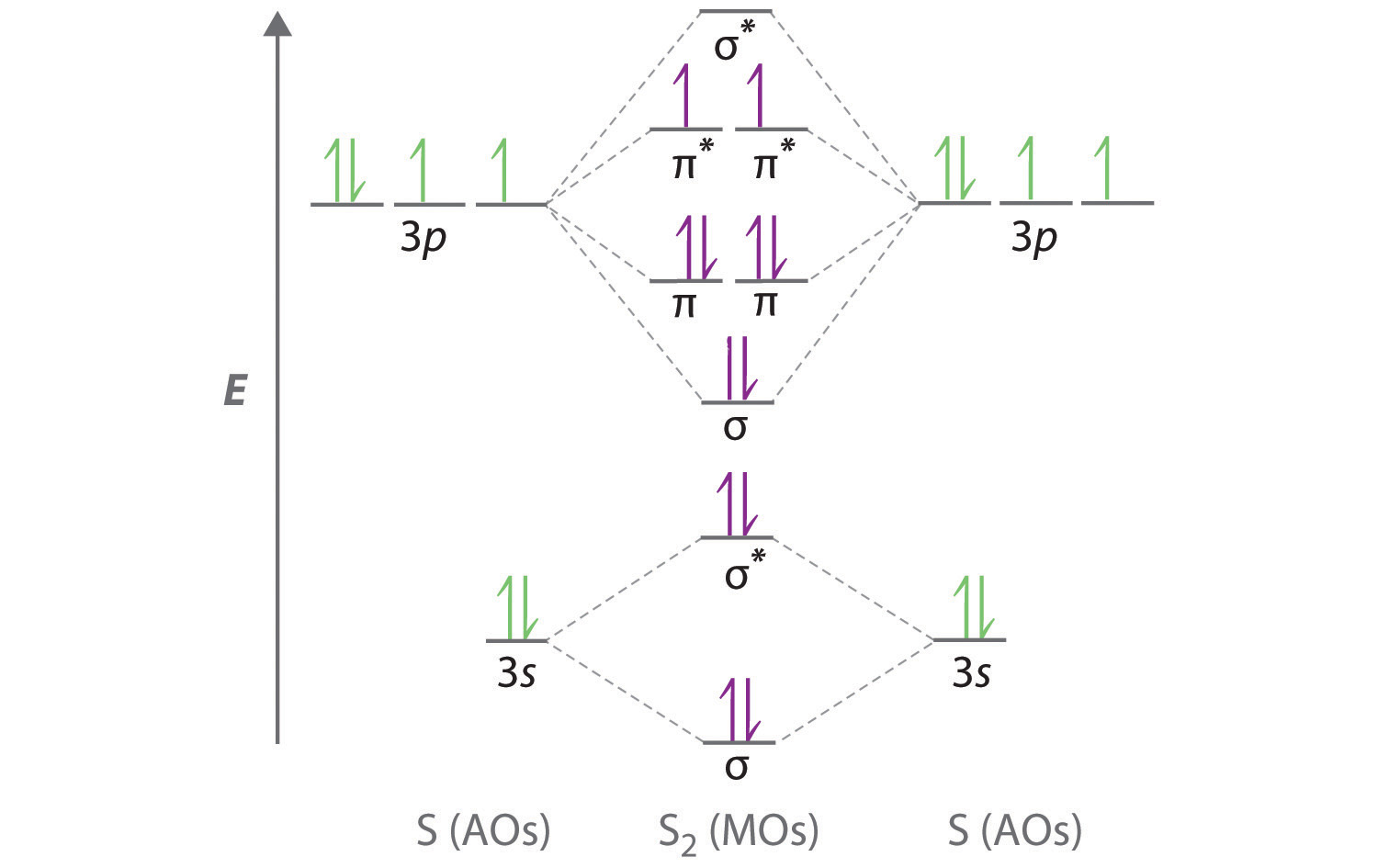
9.7: Molecular Orbitals - Chemistry LibreTexts Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
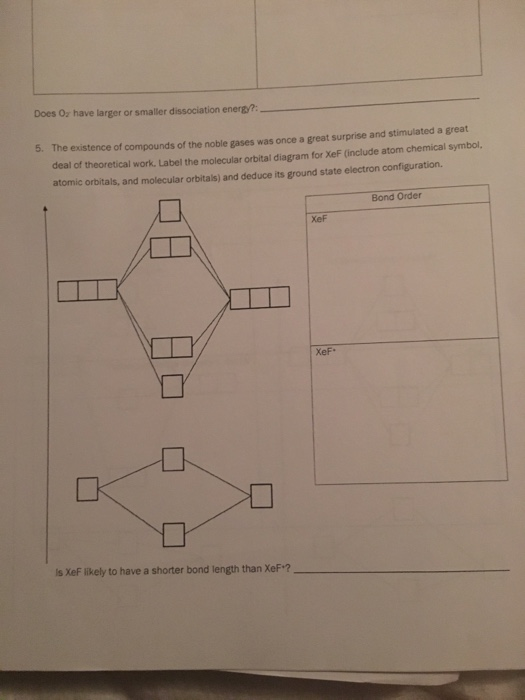
MOLECULAR ORBITALTHEORY LAB SUM 2018.pdf - MOLECULAR ... Label the molecular orbital diagram for XeF (include atom chemical symbol, atomic orbitals, and molecular orbitals) and deduce its ground state electron configuration. Is XeF likely to have a shorter bond length than XeF +? _____ Bond Order XeF XeF + 6. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic
Resonant enhancement in the valence orbital ... The nature of the resonances observed in the valence molecular orbitals photoionization cross sections of XeF 2 has been characterized using continuum multiple-scattering (MS) Xα calculations. Analysis of the theoretical cross sections of XeF 2 and a hypothetical F⋅⋅⋅F molecule with the same bond length in XeF 2 > reveals that there are at least three independent mechanisms governing ...
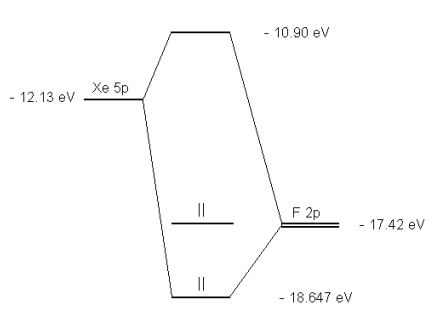
183: Semi-empirical Molecular Orbital Calculation on XeF2 ... The diagram shows two electrons each in the bonding and non-bonding orbitals for a total energy of -72.14 eV. The energy of the isolated atoms is 2 (-12.13 eV) + 2 (-17.42 eV) = - 59.1 eV. Thus, the molecule is more stable than the isolated atoms according to this crude semi-empirical model. The partial charges are calculated next.
XeF2 Lewis Structure, Molecular Geometry ... - Techiescientist When two or more atoms come together they react and combine to form homogeneous and heterogeneous molecules. This formation of molecules happens via the creation of certain bonds which hold the atoms together according to their strength. This is known as chemical bonding which is the backbone to define the internal structure and nature of a given molecular compound including the properties it exhibits( both physical and chemical). Before we jump directly into the chemical bonding of XeF2 in this article, we would like you to learn and recapitulate certain important terminologies and concepts.
Photoionization from the Xe 4d orbitals of XeF 2 (Journal ... The Xe 3d-ϵf continuum shape resonances dominate the photoionization cross sections of both the atom and molecule, but prominent resonances appear in the XeF{sub 2} cross section due to nominal excitation of Xe 3d and F 1s electrons to the lowest unoccupied molecular orbital (LUMO), a delocalized anti-bonding MO.
xef4 molecular orbital diagram - pem.pm An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of molecular orbital theory. Dear Student, a) b)In,XeF4,the central atom,Xe,has eight electrons in its outermost shell. Determine whether each is paramagnetic or diamagnetic.
halides - What is the molecular structure of xenon ... Experimental evidence. The structure of xenon hexafluoride ($\ce{XeF6}$) has always been controversial; it is a famously strong oxidising agent, so experimental studies of it have been hampered by difficulties in the isolation and storage of pure samples.Early studies indicated that the structure of $\ce{XeF6}$ showed deviations from octahedral symmetry.
MOT Molecular Orbital Diagram Of XeF2 - YouTube #MOT Molecular Orbital Diagram Of #XeF2 #Xenon Di FluoRide In English#NobleGases#InertGases#Chemistry#XenonDiFluoride#Bonding#MolecularOrbitalDiagram#Jee #Neet
Xef4(Xenon Tetrafluoride) Molecular Geometry, Lewis ... But when this atom is in an excited state, two electrons in the p-orbitals move to d-orbitals; as a result, there are four unpaired electrons in total. Out of which, two are in p-orbitals, and the other two unpaired electrons are in d-orbitals. These hybridized orbitals lead to sp3d2 hybridization in XeF4. XeF4 Molecular Geometry
Hybridization of XeF2 - Hybridization of Xe in Xenon ... There are three hybrid orbitals that contain the lone pairs and they do not form any bonds. XeF2 Molecular Geometry And Bond Angles. XeF2 molecular geometry is linear. It acquires such shape as the lone pairs present around the central atom tend to take up equatorial positions. The bond angle is said to be 180°.
Solved Although KrF+ and XeF+ have been studied, KrBr+ has ... Although KrF + and XeF + have been studied, KrBr + has not yet been prepared. For KrBr +:. a)Propose a molecular orbital diagram showing the interactions of the valence shell s and p orbitals to form molecular oribtals
PDF Lecture 4 d orbitals - University of Oxford d orbitals improve bonding, but we can explain the stability of XeF 2 without them They do not participate to the extent implied by a formal sp 3d hybridisation model unrealistic more realistic. SF 6 sp 3d2 hybrids? ionic resonance? S-F bond order = 4/6.













0 Response to "36 Xef Molecular Orbital Diagram"
Post a Comment