38 bohr diagram of sodium
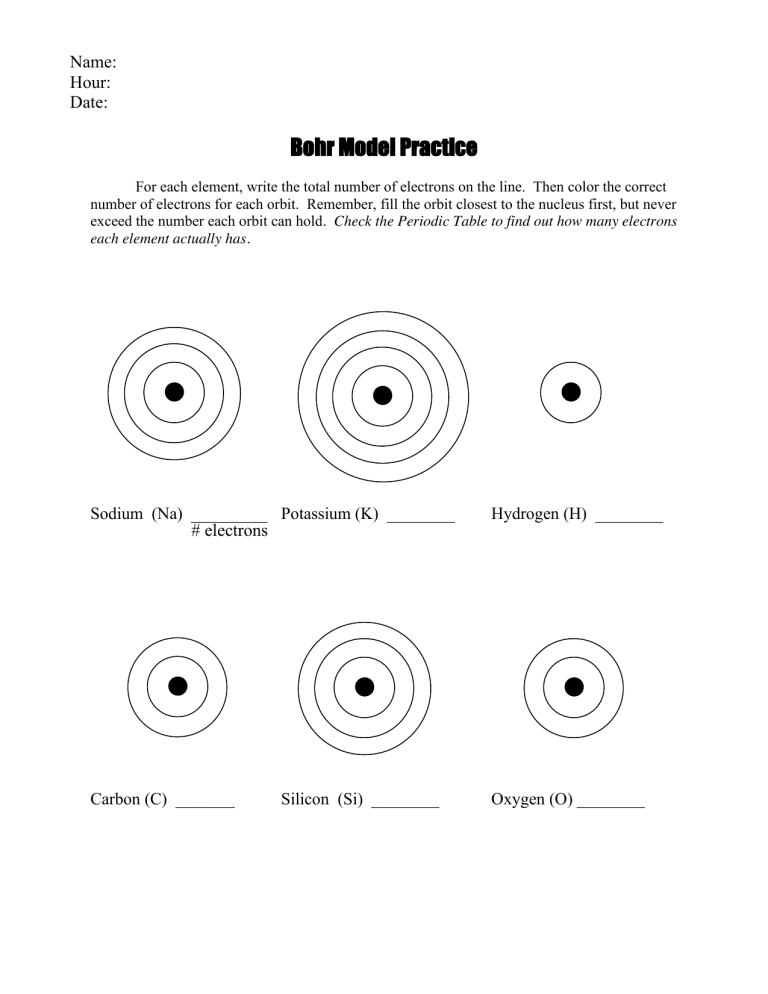
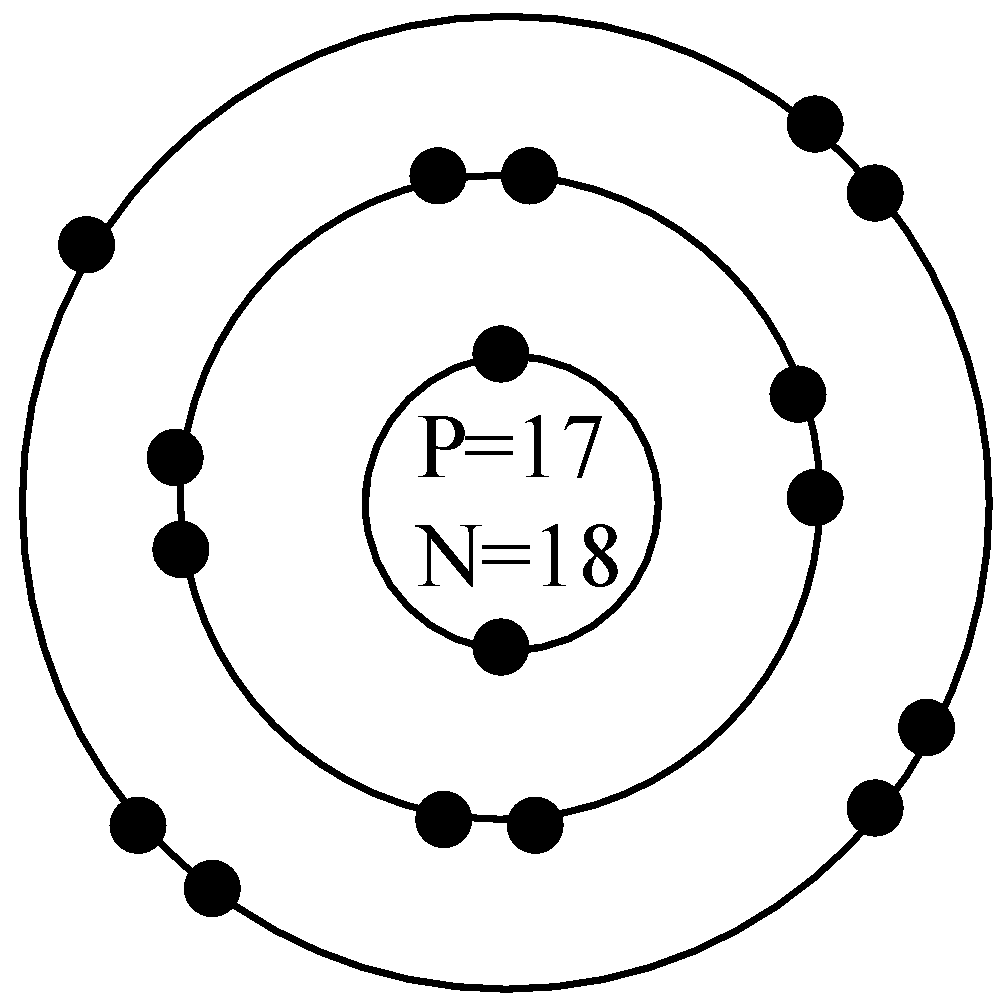
sodium atom sodium ion argon atom chlorine atom chlorine ion potassium atom potassium ion Atomic number Number of 10 protons 10 2. Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3. What do you notice about the arrangement of electrons ... March 30, 2020 - Get the detailed answer: What is the bohr model for Sodium?
The Bohr Model of Nitrogen(N) has a nucleus that contains 7 neutrons and 7 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Nitrogen contains 5 electrons that also called valence electrons.
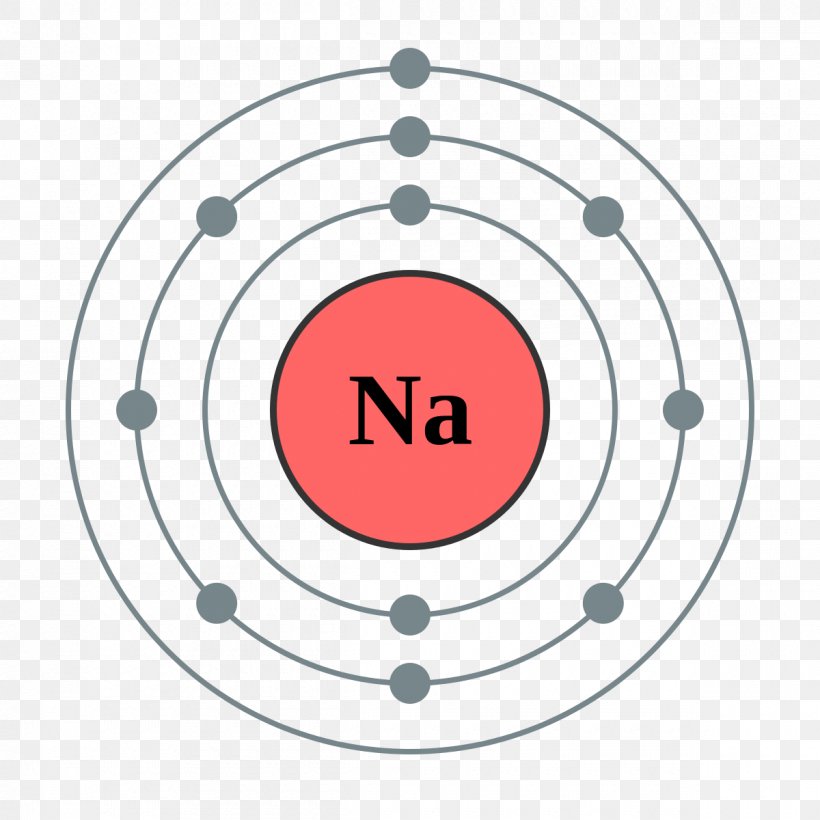
Bohr diagram of sodium
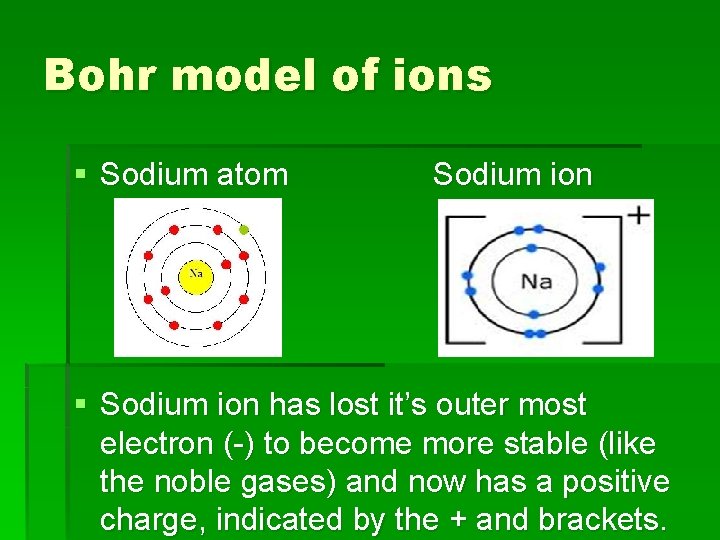
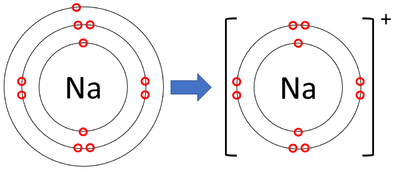
Bohr Diagram Practice Worksheet - Addressing troubles is a wonderful method to aid students boost their algebra abilities. Recognize an unidentified worth in a number sentence with an equal sign. The student must find out the worth of a number sentence utilizing fundamental math procedures. In sodium-ion, there are 11 protons but 10 electrons, i.e. sodium ion contains a lesser number of electrons. The sodium atom has only one electron in its valence shell. Sodium-ion has 8 electrons in its valence shell. Size of a sodium atom is larger than a sodium ion. Size of a sodium ion is smaller than a sodium atom. Date of Discovery: 1807 Discoverer: Sir Humphrey Davy Name Origin: soda (Na2CO3) Symbol Origin: From the Latin word natrium (sodium) Uses: medicine, agriculture Obtained From: table salts and other foods ... Note: The external links below are not a part of this site and their content is not ...
Bohr diagram of sodium. related to: sodium atom bohr model. www.3bscientific.com. Shop Our Bohr Atomic Models - World Leader In Anatomy Models. Explore Our Excellent Selection Of Bohr Atomic Models & More. Shop & Save Today! Types: Biology Supplies, Medical Simulators, Anatomical Models, Physics ... Sodium is a soft, waxy, silvery reactive metal belonging to the alkali metals that is abundant in natural compounds (especially halite). Simply so, what is a Bohr diagram? A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. Sodium(Na) is the 11th element in the periodic table and its symbol is 'Na'. This article gives an idea about the electron configuration of sodium and orbital diagram, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles.Hopefully, after reading this article you will know in detail about this. Complete step – by – step answer: The concept of chemistry which deals with the topic called some basic concept of chemistry tells us about the various models for atoms given by the scientists among which the Bohr’ model is the one. Now we shall see how to draw the Bohr diagram for sodium ...
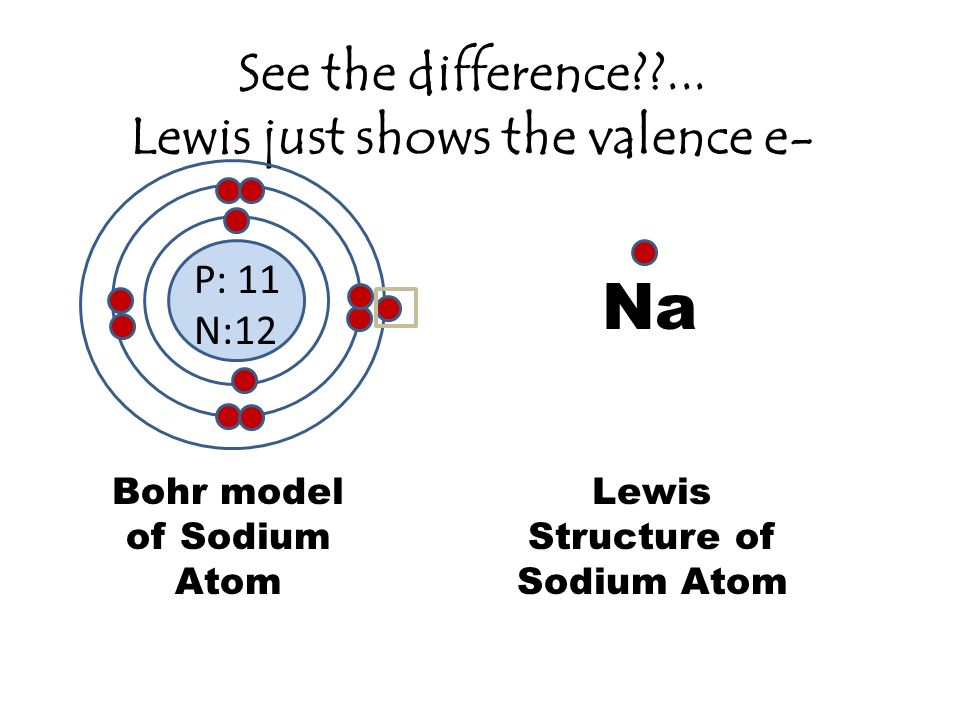
August 16, 2020 - In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. Theoretically, they would be more energetically stable if they followed the octet rule and had eight. ... Bohr diagrams indicate how many electrons fill each principal shell. Bohr model of Sodium Atom. Lewis Structure of Sodium Atom. Na. Lewis Dot Structures. Lewis dot structures are really simple - they are just the valence e- represented as dots around an element. 2 electrons together is called a . lone pair. The # of valence e- is … 8. 8 e- is stable. Draw the Bohr Models showing all the electrons in each energy level. 1. Magnesium compounds are used in the production of uranium for nuclear reactors. Draw the Bohr model for magnesium. Niels Bohr 2. Sodium is found in salts that can be used to seed clouds to increase rainfall. Draw the Bohr model for sodium. Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
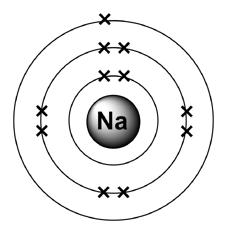
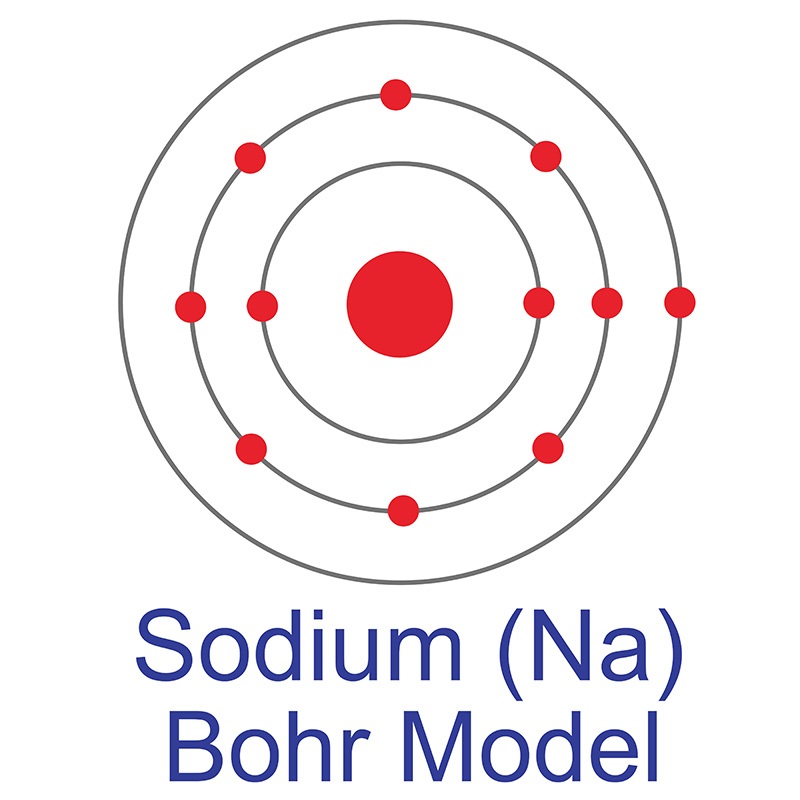
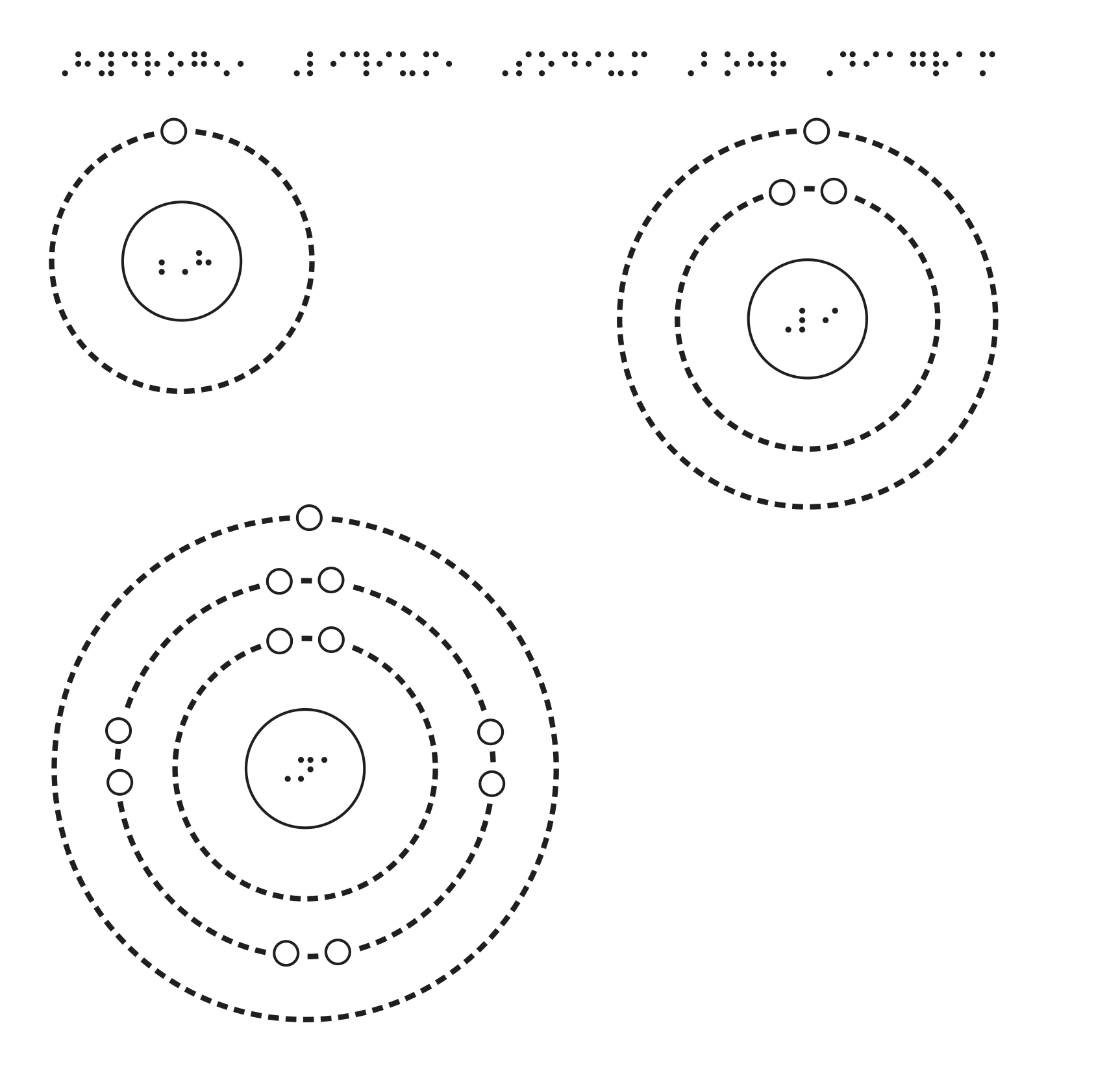
Sodium has 2 electrons in its first shell, 8 in its second and 1 in its third.Check me out: http://www.chemistnate.com Isotopes of sodium. There are thirteen recognized isotopes of sodium. 23 Na is the only stable isotope. As such, it is considered a monoisotopic element and it has a standard atomic mass: 22.98976928 (2) u. Sodium has two radioactive cosmogenic isotopes ( 22 Na, half-life = 2.605 years; and 24 Na, half-life ≈ 15 hours). February 19, 2016 - The first and second valence shells are completely full, since their 2 and 8 electrons only take up the first 10 of sodium's 11 electrons. Thus there will be 1 leftover electron in the third valence shell, so the Bohr diagram of "Na" can be drawn as follows: We can follow a similar process ... Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of Aluminum (Al) 2, 8, 3: 14: Bohr model of Silicon (Si) 2, 8, 4: 15: Bohr model of Phosphorus (P) 2, 8, 5: 16 ...
A Lewis dot structure is like a simplified Bohr-Rutherford model. The Lewis Dot diagram contains the element symbol with dots representing electrons. The only electrons shown are those on the outer energy level or valence electrons. The electrons are placed around the element symbol, one at a time, clockwise or counterclockwise, and then ...
The shell closest to the nucleus is called the K.Bohr rutherford diagram of aluminum further atomo furthermore file 20 calcium ca enhanced bohr model along with bohr model drawing of oxygen in addition bohr rutherford diagram for aluminum in addition sodium lab eng moreover bohr rutherford diagram as well as electron shell diagram.
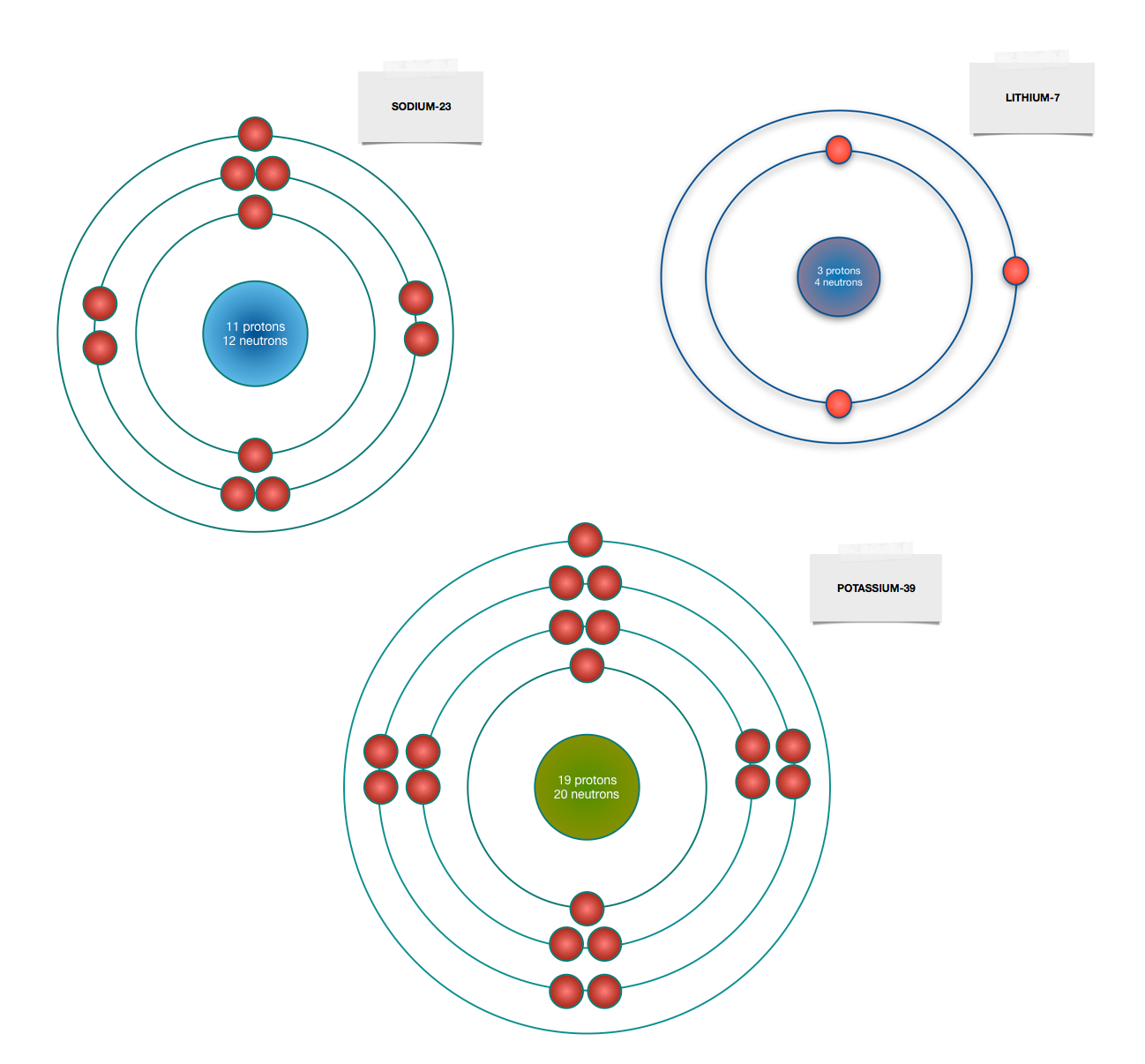
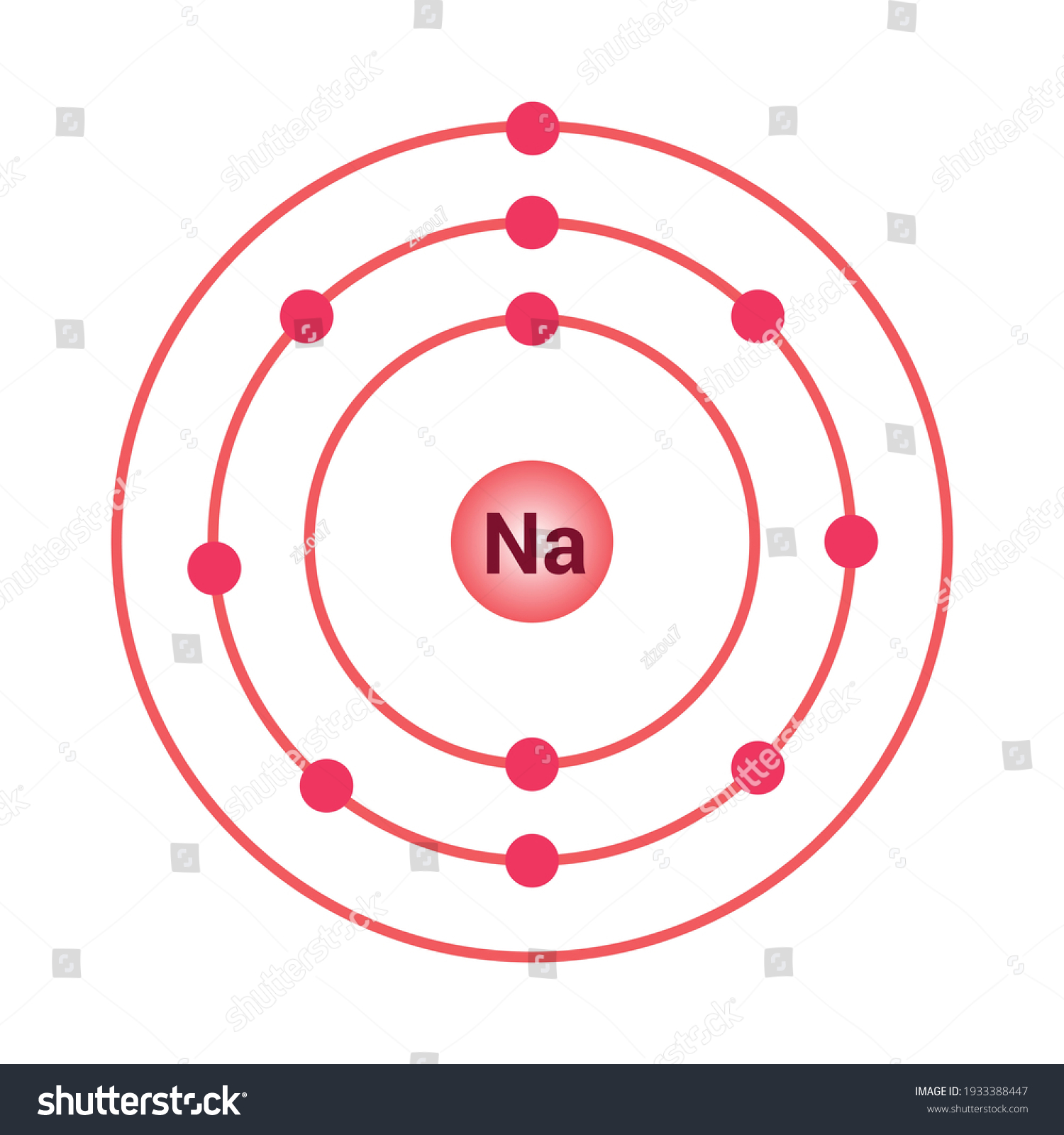
Sodium (Na). Diagram showing the nuclear composition and electron configuration of an atom of sodium-23 (atomic number: 11), the most common isotope of the element sodium.
Nov 13, 2018 · In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. Theoretically, they.A Bohr diagram depicts an atom with a small, central nucleus and the electrons in their valence shells. The first valence shell contains 2 electrons, and the second and third shell have 8 electrons each, and the number keeps growing.
Argon Bohr Model — Diagram, Steps To Draw. Argon is a group 18th element having atomic number 18. It is denoted by the symbol Ar and is a noble gas. It is also the third most abundant gas present in the atmosphere of the earth. Around 1.288% by mass of argon is present in the air, which can be isolated through fractional distillation.
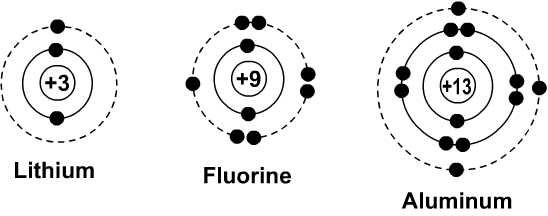
draw a Bohr Diagram for a Sodium atom and an Aluminum atom. SUM IT UP! atom. 16 pt 1. This is a Bohr Diagram of a 16 no 2 How many shells does this atom have? 15. He 3 What is the shell closest to the nucleus called? jenkinsxavier is waiting for your help. Add your answer and earn points.
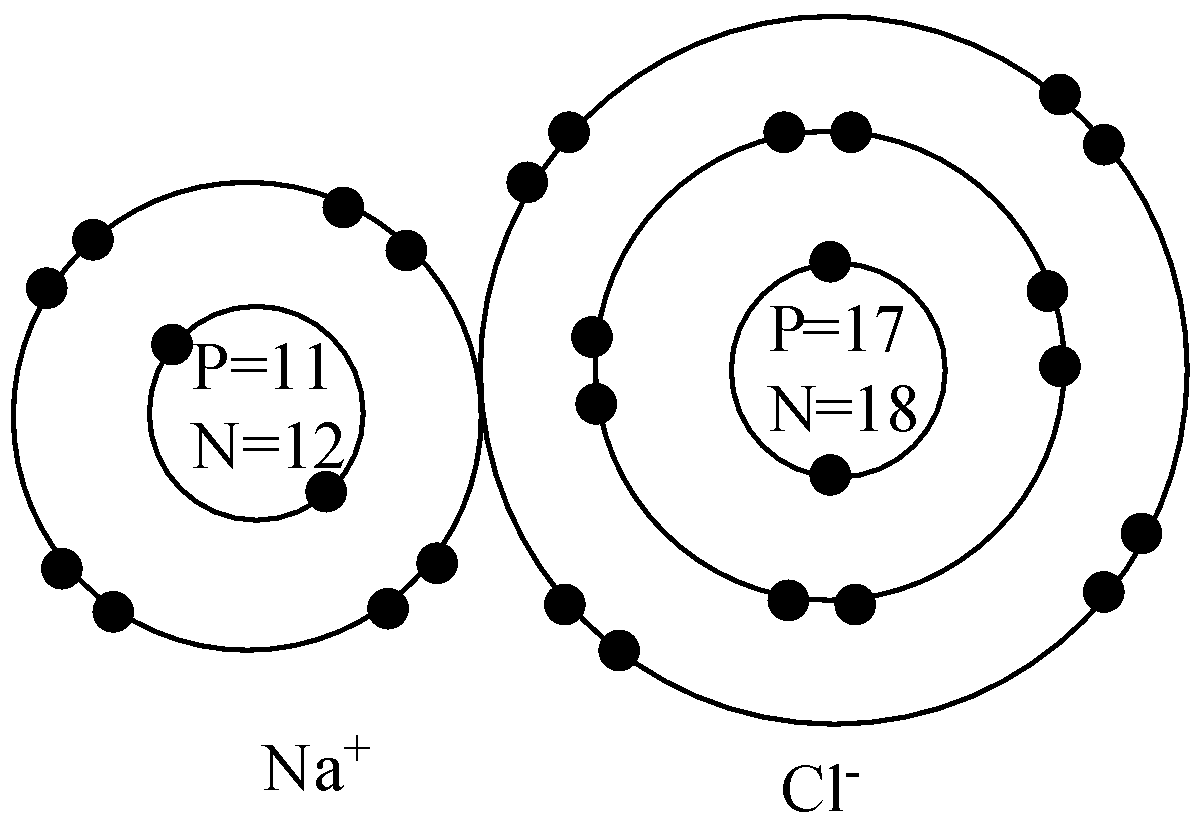
The sodium atom wants to lose an electron and the chlorine atom wants to gain an electron. When the two atoms come together the electron from the sodium atom jumps into the gap in the outer shell of the chlorine atom. If you look at the diagram the sodium . ion. now contains only ten electrons and the new chloride ion (an anion) has eighteen ...
The exercises present in Chapter 4 of NCERT Solutions for Class 9 Science are –. Number 4.1 – Charged particles in matter 2 Questions ( 2 short) Number 4.2 – The structure of an atom 4 Questions ( 4 short) Number 4.2.4 – Neutrons 2 Questions ( 2 short) Number 4.3 – How are electrons distributed.
March 10, 2012 - Valence Electrons • The electrons ... the valence shell • The number of electron shells with electrons is the same as (=) the period number • Atoms will try to gain or lose electrons to have a full valence shell · 5. Bohr Diagrams 1) Find your element on the periodic ...
Bohr-Rutherford diagram of The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2. Both elements have three electron shells. Sodium has one electron in its outer shell and chlorine has seven. Neither of them has an outer shell that is filled. The atomic number of Na is 11, so it has 11 electrons. The first and Now, we must draw the Bohr diagram for the NaCl model.
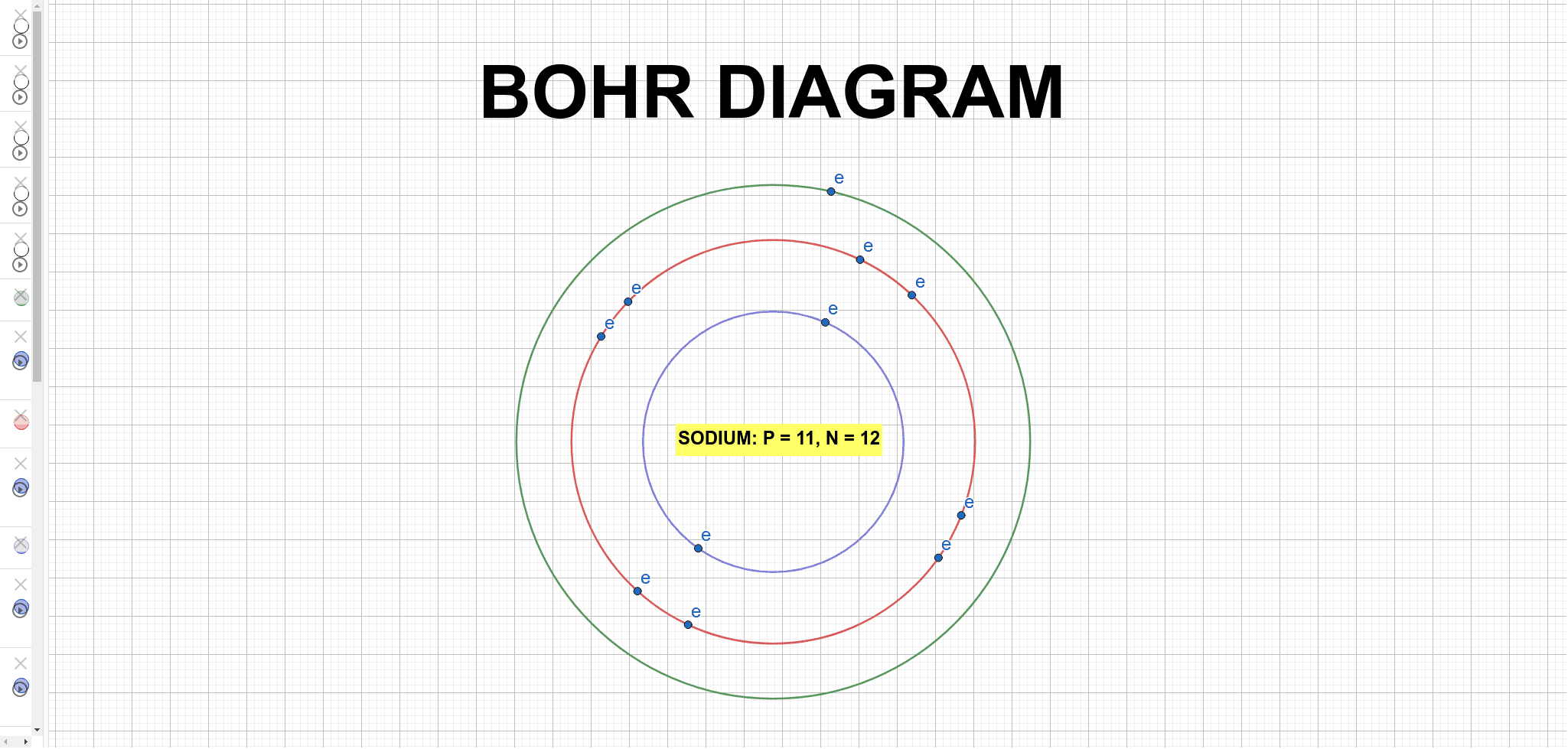
3 weeks ago - The Bohr Model of Sodium(Na) has a nucleus that contains 12 neutrons and 11 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Sodium contains only 1 electron that also called valence electron.
April 27, 2018 - An effective learning method is an interactive hands-on approach to chemistry by crafting 3D models of atoms, in this case sodium., using readily available craft materials.
July 10, 2021 - Sodium Bohr Model What is the Bohr diagram for sodium? Answer and explanation: A Bohr model for sodium shows it has eleven protons and neutrons in its nucleus, with eleven electrons orbiting the earth at three energy levels.How large is the number of electrons in sodium in relation to this?11We ...
Students will understand Bohr’s experimental design and conclusions that lead to the development of his model of the atom, as well as the limitations of his model.
30 seconds. Q. What best describes the difference between the bohr model and the lewis dot? answer choices. The bohr model diagram represents all the subatomic particles, while the lewis dot diagram only shows the symbol and the valence electrons. The lewis dot shows all the electrons and the bohr model only shows the electrons in the last shell.
Sodium Bohr Model — Diagram, Steps To Draw Sodium is a highly reactive metal element. It has the atomic number 11 and is represented by the symbol Na. It belongs to group 1A of the periodic table and hence, is an alkali metal. It is silvery-white in appearance and exists in nature in the form of minerals such as sodalite, rock salt, feldspar, etc.
Since Sodium's Atomic Number is 11, that is also the number of electrons. The first energy level can hold 2 electrons, the next 8, and the third 18. So the diagram has two electrons on the first ...
September 27, 2015 - Sep 14, 2012 - This Pin was discovered by Jackie B.. Discover (and save!) your own Pins on Pinterest
AboutPressCopyrightContact usCreatorsAdvertiseDevelopersTermsPrivacyPolicy & SafetyHow YouTube worksTest new features · © 2022 Google LLC
A Bohr model for sodium shows that it has eleven protons and neutrons inside the nucleus, with its eleven electrons orbiting in three energy levels....
Is there a simple privacy law that actually makes sense? The atomic number of #"Na"# is #11# , so it has #11# electrons. Sodium has one electron in its outer shell and chlorine has seven. draw a Bohr-Rutherford diagram for helium. 2. Bohr-Rutherford diagram of The bohr Rutherford diagram for ...
Bohr-Rutherford Diagram of NaCl (sodium chloride, table salt) - YouTube NaCl, sodium chloride, is an IONIC compound, which means electrons are TRANSFERRED from one atom to another.You'll have to...
Bohr-Rutherford Diagram: Sodium is the cation as it has a positive charge after losing one electron, while fluoride is the anion as it has a negative charge after gaining one electron. The two atoms attract, as they have opposite charges. Lewis Structural Diagram- It is a linear shape and there is a single bond. Next Page
Example: Determine the formula of a compound formed by the reaction of sodium and fluoride. Solution: First examine the electron arrangement of the sodium and fluorine atoms. Symbol: Atomic No. Bohr diagram: Group No. Lewis Dots: Na: 11: 2 - 8 - 1: 1: 1: F: 9: 2 - 7: 7: 7: Write the Lewis symbol for each atom. See Graphic on the left.
Now look carefully at the following Bohr models of sodium and chlorine.
Nov 19, 2019 - This Pin was discovered by Christy Sego. Discover (and save!) your own Pins on Pinterest.
Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. This is because protons and neutrons ...
Bohr model for sodium atom? - Answers A Bohr model is constructed by drawing a circle in the middle. Then a ring around it. On that ring, put 2 dots. The same with the second ring. contains a...
Date of Discovery: 1807 Discoverer: Sir Humphrey Davy Name Origin: soda (Na2CO3) Symbol Origin: From the Latin word natrium (sodium) Uses: medicine, agriculture Obtained From: table salts and other foods ... Note: The external links below are not a part of this site and their content is not ...
In sodium-ion, there are 11 protons but 10 electrons, i.e. sodium ion contains a lesser number of electrons. The sodium atom has only one electron in its valence shell. Sodium-ion has 8 electrons in its valence shell. Size of a sodium atom is larger than a sodium ion. Size of a sodium ion is smaller than a sodium atom.
Bohr Diagram Practice Worksheet - Addressing troubles is a wonderful method to aid students boost their algebra abilities. Recognize an unidentified worth in a number sentence with an equal sign. The student must find out the worth of a number sentence utilizing fundamental math procedures.

















0 Response to "38 bohr diagram of sodium"
Post a Comment