38 What Does Each Box In An Orbital Diagram Represent
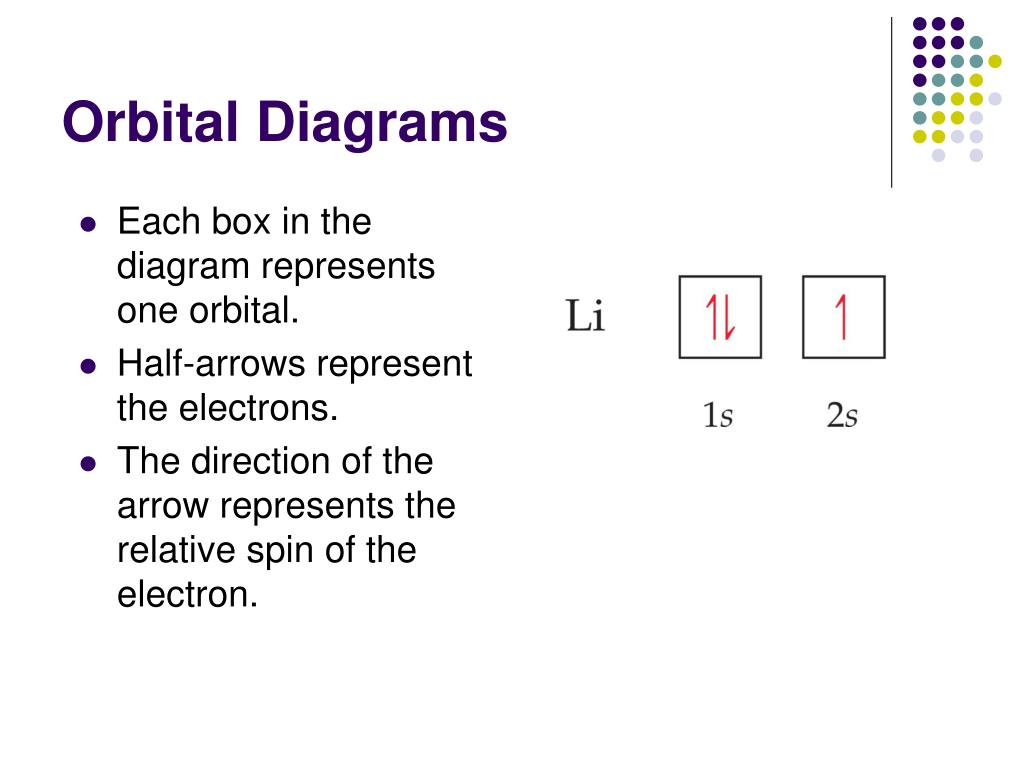
Chapter 7 - Each box in the diagram represents one orbital. - Half-arrows represent the electrons - Direction of the arrow represents. the relative spin of the 76. Example writing orbital diagrams. Write an orbital diagram for silicon. SOLUTION Since silicon is atomic number 14, it has 14 electrons. Orbital Box Diagrams. - ppt download 2 Orbital (Box) Diagrams Orbital Diagram = collapsed version of the orbital filling diagram. Example: Carbon (Z = 6. therefore e- = 6) 1 s s p Note: Each box = an orbital Arrows = electrons. 3 Each sub-level has a different number of boxes that represents the number of orbitals in each.
What does each box in a orbital diagram represent? - Answers The fuse box diagram is the diagram on the back panel of the fuse box cover. It shows what each fuse and relay goes to, and what it helps. What percentage does each section of a box and whisker plot represent?

What does each box in an orbital diagram represent
PDF Orbital Diagrams Chem Worksheet An orbital diagram use Each box in an orbital diagram represents an orbital. Orbitals have a capacity of two electrons. Arrows are drawn inside the boxes to represent electrons. In writing an orbital diagram the first step is to determine the. Boxes drawn for various sublevels number of electrons. Normally this is the... What Is the Orbital Diagram for Sulfur? The boxes represent sulfur's orbitals. An orbital diagram illustrates how the electrons pair off in each orbital. Hund's Rule states that one electron must be placed in each orbital of a particular energy level before a second electron is placed in the same orbital. (Get Answer) - What does each box in an orbital diagram represent?. What does each box in an orbital diagram represent? Feb 05 2021 01:58 PM.
What does each box in an orbital diagram represent. What does each box in an orbital diagram represent? 9 hours ago Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies 7 hours ago The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is #2#... What does an orbital represent? What does each orbital or shell actually represent? Even though we know that electron shells do not actually consist of electrons travelling in neat circular orbits around the nucleus, they are real enough in the sense that A picture of an orbital represents the region near the nucleus that the electrons orbit. 6.4 Electronic Structure of Atoms (Electron Configurations) - Chemistry However, this pattern does not hold for larger atoms. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows... 6.6: 3D Representation of Orbitals - Chemistry LibreTexts To understand the 3D representation of electronic orbitals. An orbital is the quantum mechanical refinement of Bohr's orbit. Because Ψ2 gives the probability of finding an electron in a given volume of space (such as a cubic picometer), a plot of Ψ2 versus distance from the nucleus (r) is a plot of the...
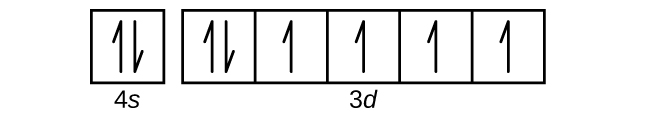
Orbital Diagrams Chemistry Tutorial An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below). Solved: Ments Part C What Does Each Box In An Orbital Diag... Ations A Shell An Electron An Orbital Se Course An Atom Ses Submit Request Answer DY Evals Part D What Quantity Is Represented By The Half Arrows In An Orbital Diagram? Orbital Diagrams and Electron Configuration - Basic Introduction... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram... Orbital Diagrams — Overview & Examples - Expii An orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron Electron configurations do not show orbital energies or electron pairing. For each electron in a subshell, place an arrow in its box. An up arrow represents a spin-up electron.
s p d f obitals notation shapes diagrams how to work out electron... How do you work out the electron configuration of an atom. Parts 2.1 and 2.2 This page is an 'electronic' introduction to the structure of the modern Also in the table, some are written out in box diagram format, each box represents an orbital with a maximum of two electrons of opposite spin... electronic structure and atomic orbitals Orbits and orbitals sound similar, but they have quite different meanings. Note: In this diagram (and the orbital diagrams that follow), the nucleus is shown very much larger than it really is. Soon afterwards, you do the same thing, and find that it is in a new position. You have no idea how it got... How to Do Orbital Diagrams | Sciencing Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and Orbital diagrams use the same basic format, but instead of numbers for the electrons, they use ↑ and ↓ arrows, as well as giving each orbital its own... Welcome to CK-12 Foundation | CK-12 Foundation The orbital diagram in does obey Hund's Rule, because the two electrons in the singly occupied orbitals have the same spin. Orbital diagrams are drawn by representing each orbital as a box, each 'spin-up' electron in an orbital as an upward pointing arrow in the box, and each 'spin-down'...
What does each box in an orbital diagram represent? | bartleby Q: From the partial (valence-level) orbital diagram, write the ground-state electron configuration and ... Q: For each pair, identify the orbital in which an electron possesses more energy. (a) 4s or 5s; (b) 5p...
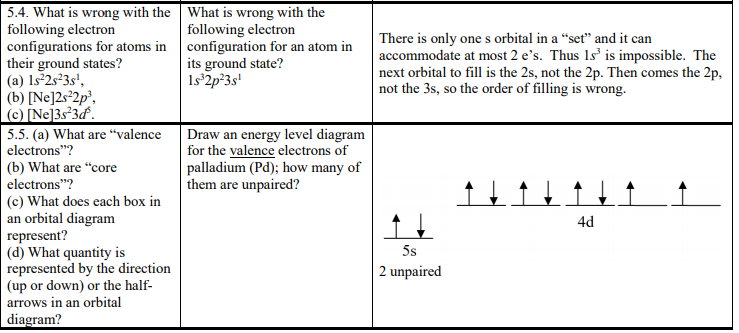
General Chemistry I - Tutorials 2 and 3 | Atomic Orbital | Atoms (c) What does each box in an orbital diagram represent? (d) What quantity is represented by the half arrows in an orbital diagram? 14. Write the condensed electron configurations for the following atoms, using the appropriate noble-gas core abbreviations: (a) Cs, (b) Ni, (c) Se, (d) Cd, (e) U, (f) Pb.
What orbital diagrams represent the electron... - Quora Each s orbital is spherically symmetric about the origin. The energy of an atomic orbital depends upon kinetic energy of the electron plus potential energy that arises from its electrostatic interactions with nucleus and another electrons; It...
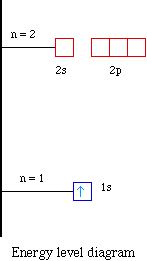
Orbital Diagrams & Electron Configurations for Atoms and Ions ...orbitals. • boxes or lines represent each orbital • arrows within boxes represent the electrons • max two per box Draw an orbital diagram for beryllium (Z=4) 1s Guidelines for drawing orbital diagrams 1s 1 in an oxygen atom? b) How many e- do I add/remove? c) What's the new total number of e
SOLVED:(a) What are "valence electrons"? (b) What are... ...does each box in an orbital diagram represent? (d) What quantity is represented by the direction (up or down) of the half-arrows in an orbital diagram? configuration of nearest noble gas element option C. Each box in an orbital diagram, so each box in orbital tig Ron represents, and barb
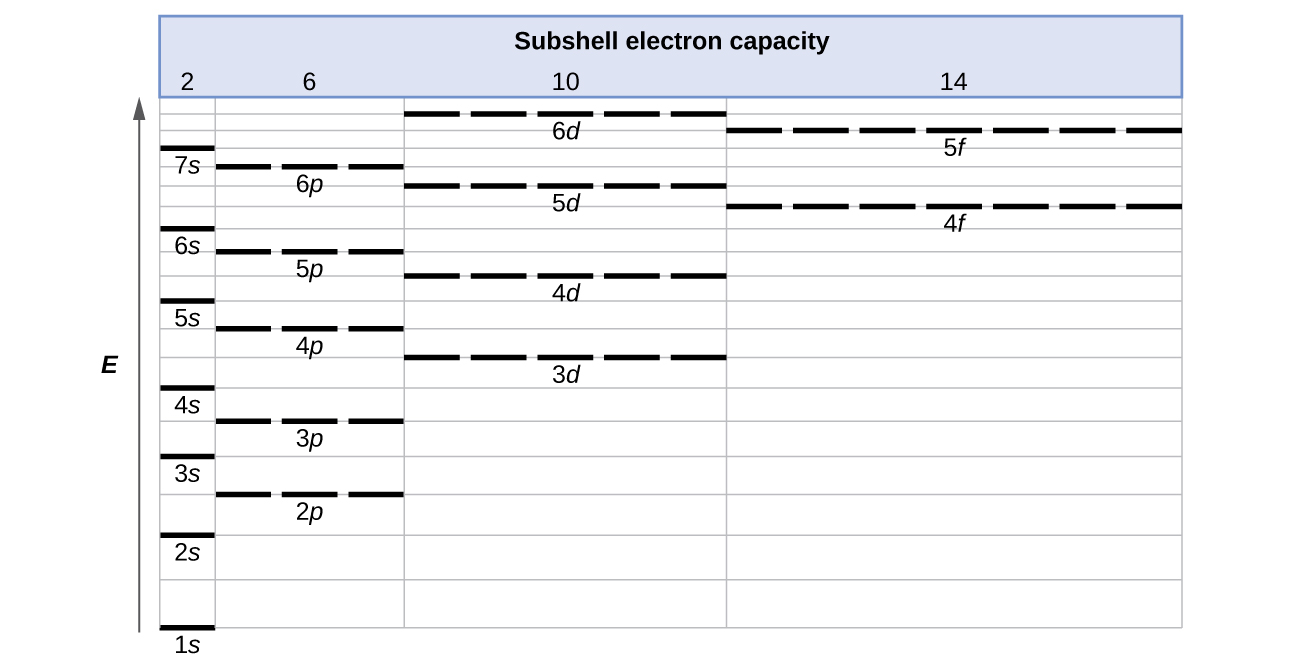
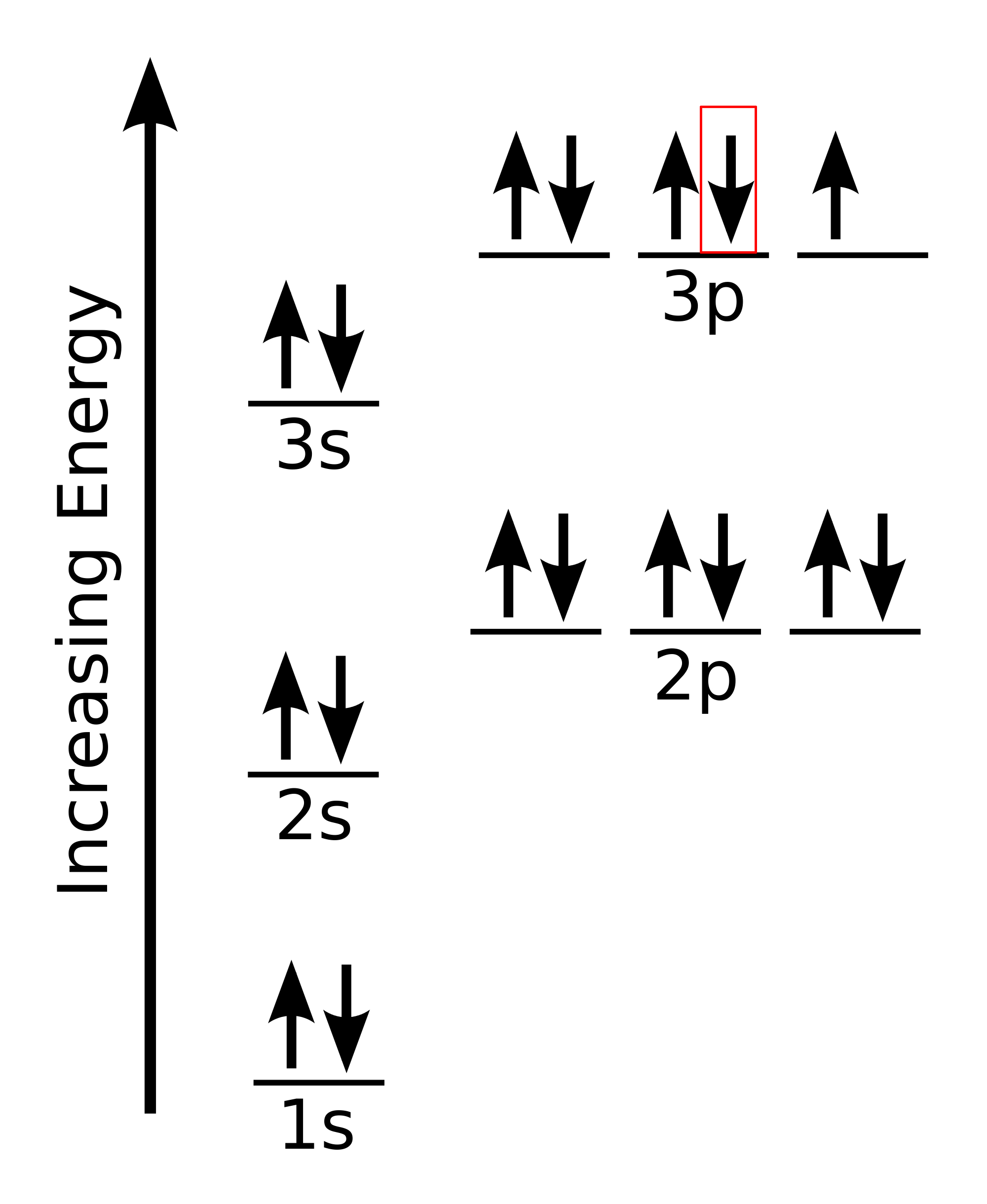
6.4 Electronic Structure of Atoms (Electron Configurations) - Chemistry Orbital diagrams are pictorial representations of the electron configuration, showing the individual For orbital diagrams, this means two arrows go in each box (representing two electrons in each However, we do find exceptions to the order of filling of orbitals that are shown in Figure 3 or Figure 4...
1.10 Orbital Diagrams - Week 1 | Coursera We represent that box to represent the orbital that the electron is occupying. If we were to assign the quantum numbers for those electrons, actually we'll do that here in a little bit. Okay? So each electron is going to have a set of four quantum numbers.
Electronic Structure of Atoms (Electron Configurations) | Chemistry for... For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite However, we do find exceptions to the order of filling of orbitals that are shown in Figure 3 or Figure 4. For instance, the electron configurations (shown in...
Atomic orbital - Wikipedia Each orbital in an atom is characterized by a set of values of the three quantum numbers n, ℓ, and ml, which respectively Fundamentally, an atomic orbital is a one-electron wave function, even though most electrons do not exist in one-electron atoms, and so the one-electron view is an approximation.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical The sign of the phase itself does not have physical meaning except when mixing orbitals to form The vertical axis always represents the orbital energies. Each atomic orbital is singly occupied with an...
what does each box represent in an orbital diagram | Quizlet boxes with arrows represent the electrons in an atom, each box in an orbital diagram represent an orbital. Each box represents an orbital which can accommodate 2 e's.
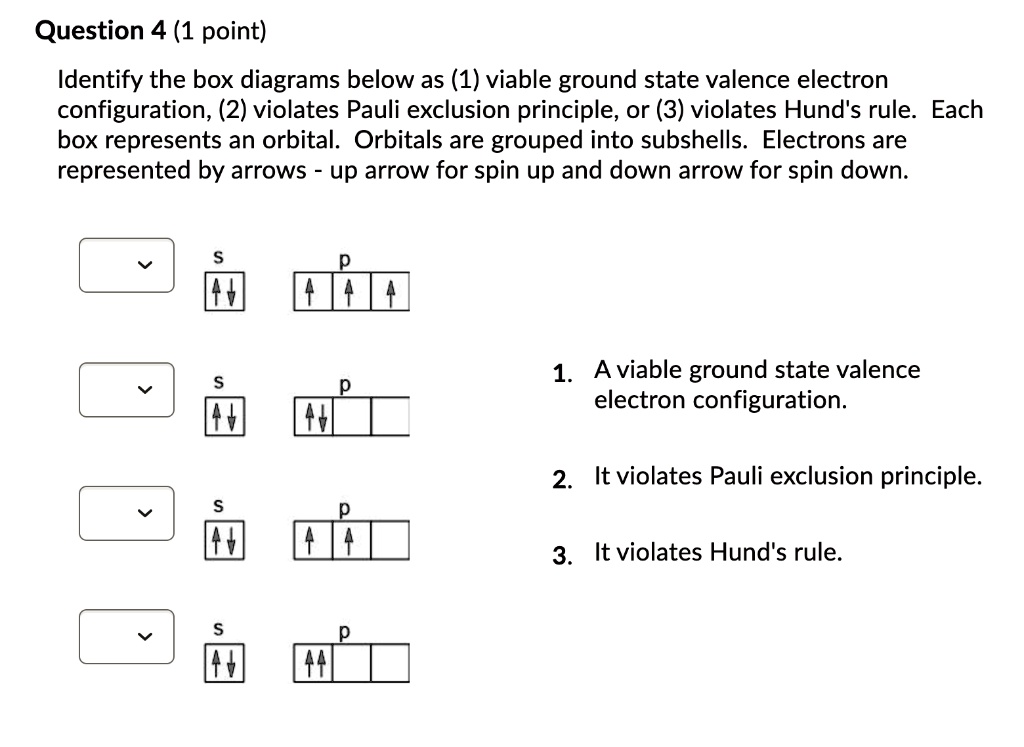
Ninth grade Lesson Electron Orbital Diagrams | BetterLesson SWBAT complete electron orbital diagrams (using boxes and arrows) following Aufbau Principle, Hund's Rule, and the Pauli Exclusion Principle. Today's Warm-Up: "What do the arrows represent in an electron orbital diagram?" In this case, the warm-up is asking students to call upon information...
(Get Answer) - What does each box in an orbital diagram represent?. What does each box in an orbital diagram represent? Feb 05 2021 01:58 PM.
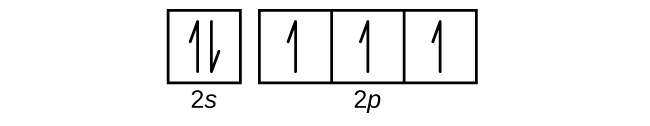
What Is the Orbital Diagram for Sulfur? The boxes represent sulfur's orbitals. An orbital diagram illustrates how the electrons pair off in each orbital. Hund's Rule states that one electron must be placed in each orbital of a particular energy level before a second electron is placed in the same orbital.
PDF Orbital Diagrams Chem Worksheet An orbital diagram use Each box in an orbital diagram represents an orbital. Orbitals have a capacity of two electrons. Arrows are drawn inside the boxes to represent electrons. In writing an orbital diagram the first step is to determine the. Boxes drawn for various sublevels number of electrons. Normally this is the...


![SOLVED:Question 5: [5 marks] Consider the following orbital ...](https://cdn.numerade.com/ask_images/a0b37034d60d436b933ae4c31faaf5dc.jpg)






















0 Response to "38 What Does Each Box In An Orbital Diagram Represent"
Post a Comment