Simple steps for drawing the Lewis dot structure for CH4 — Methane (CH4) lewis dot structure, molecular geometry, electron geometry, polar or nonpolar, bond ...Molecular geometry/shape of CH4: TetrahedralThe formal charge of CH4: 0Total Valence electron for CH4: 8Name of Molecule: Methane The Lewis diagram is drawn by showing valence electrons in the form of dots drawn around the atom and lines predicting the bond formation. These lines also ...Dec 17, 2020 · Uploaded by Wayne Breslyn
For CH4 you have a total of 8 total valence electrons. Drawing the Lewis structure for CH4 (named methane) requires only single bonds. It's one of the easier ...Oct 28, 2016 · Uploaded by Wayne Breslyn

Dot diagram for ch4
This is the final Lewis Dot Diagram. The pairs of dots represent shared electrons and you can see that the Carbon possesses 8 and the Hydrogens possess 2 each.1 answer · 1 vote: I will explain this with pictures, and some captions. This is just the five atoms in ... solution. expand. Solve any question of Chemical Bonding and Molecular Structure with:- ... The Lewis dot diagram representing ammonia molecule is:.1 answer · Top answer: This is correct because it shows the each electron of C and hydrogen that are combining and shows that there are 8 electrons in the valence shell of the ...
Dot diagram for ch4. solution. expand. Solve any question of Chemical Bonding and Molecular Structure with:- ... The Lewis dot diagram representing ammonia molecule is:.1 answer · Top answer: This is correct because it shows the each electron of C and hydrogen that are combining and shows that there are 8 electrons in the valence shell of the ... This is the final Lewis Dot Diagram. The pairs of dots represent shared electrons and you can see that the Carbon possesses 8 and the Hydrogens possess 2 each.1 answer · 1 vote: I will explain this with pictures, and some captions. This is just the five atoms in ...

How to draw the lewis dot structure of Methane (CH4)
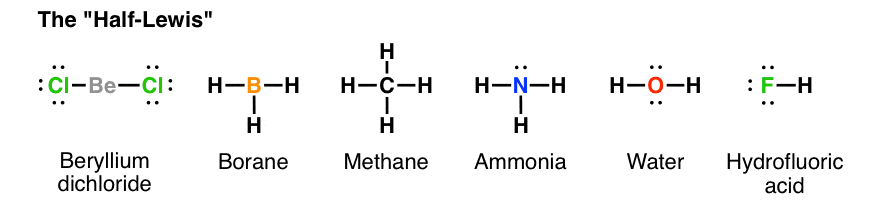
From Gen Chem to Org Chem, Pt. 7 - Lewis Structures – Master ...
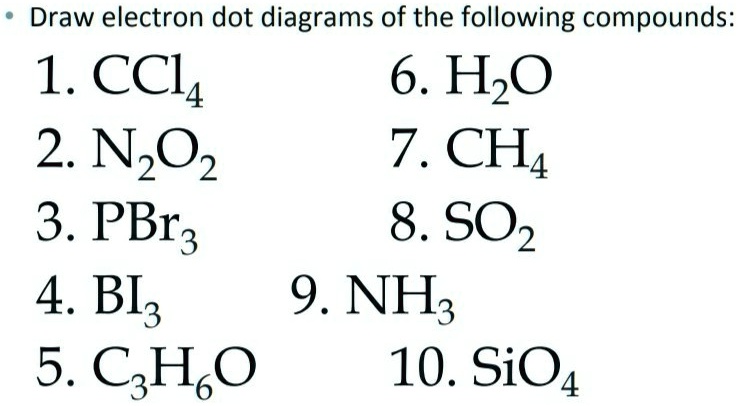
SOLVED:Draw electron dot diagrams of the following compounds ...
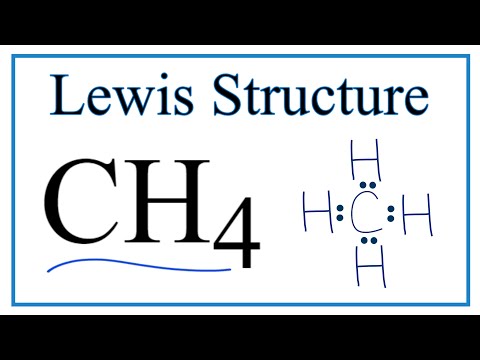
Lewis Dot Structure Practice Pdf - 01/2022

Lewis Dot Structure …for CH4 - ppt download

CH4 lewis structure, Molecular geometry, Polar or nonpolar ...
CH4 Methane Covalent Bonding .Methane Formula Diagram Design ...

What is the Lewis structure of methane? | Study.com
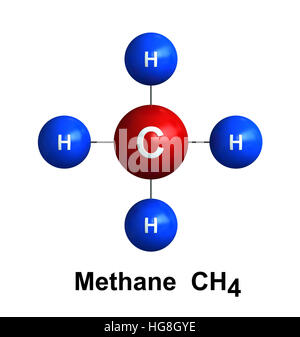
Methane (CH4) gas molecule, chemical structure. Main ...

Quiz & Worksheet - Lewis Structures | Study.com

Molecular Geometry | CK-12 Foundation

Lewis Dot Structure - Easy Hard Science

How to Draw the Lewis Structure of CH4 (methane) - YouTube
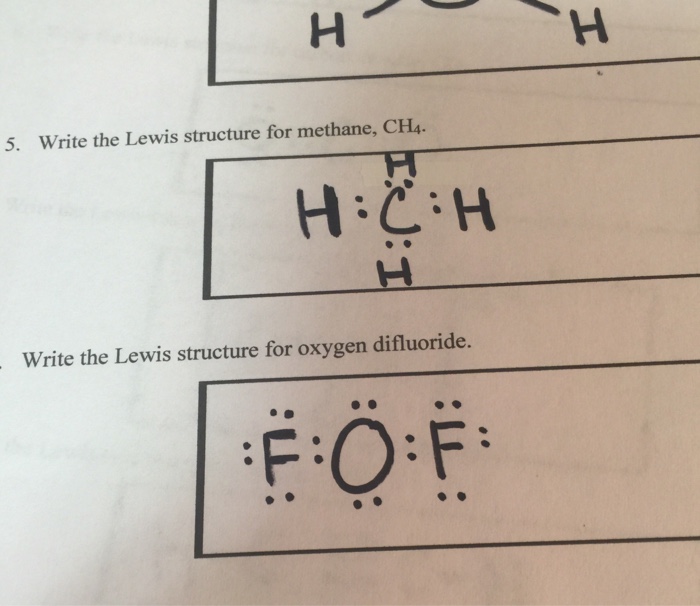
Solved 5. Write the Lewis structure for methane, CH4 Write ...
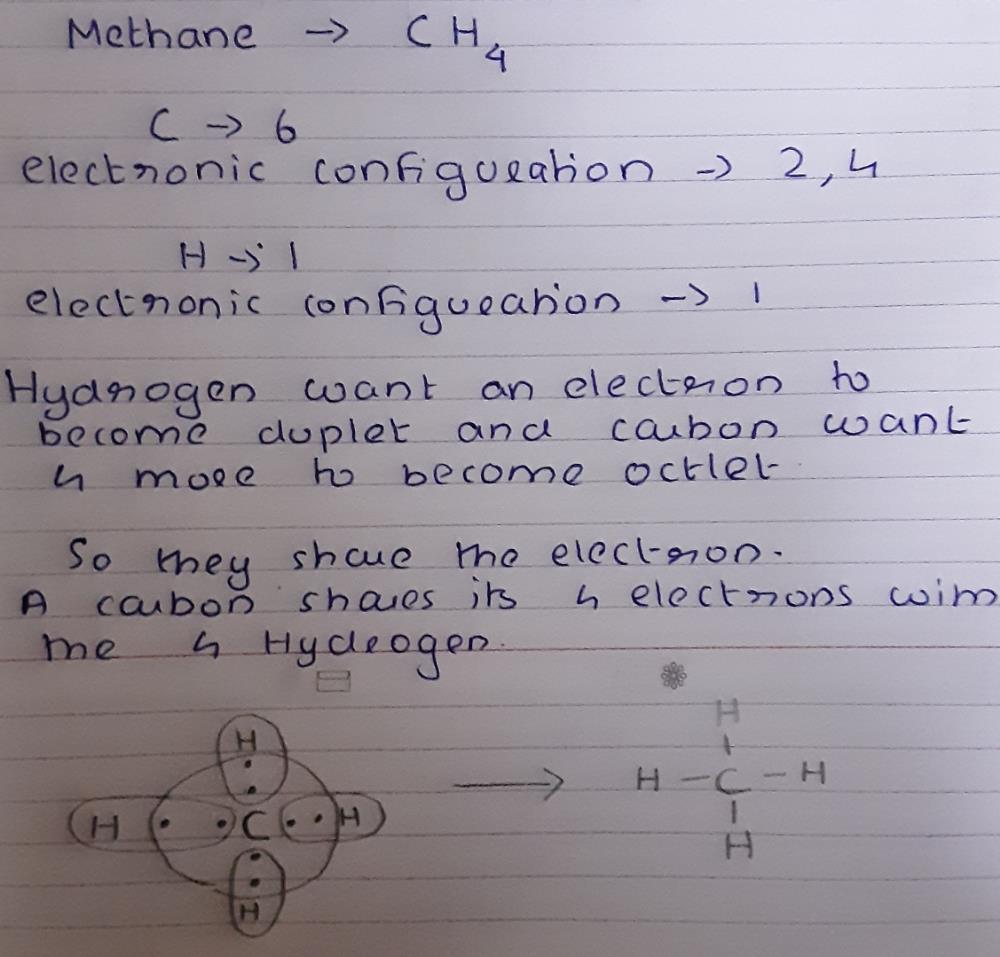
Draw the electron dot diagram of a methane molecule [C=6,H=1 ...

draw the electron dot structure of CH4 - Brainly.in

What type of bonds are present in the following molecules ...

How to determine the Lewis structure for CH4 - Quora

1) Draw the electron dot diagram of chemical bonds in methane ...
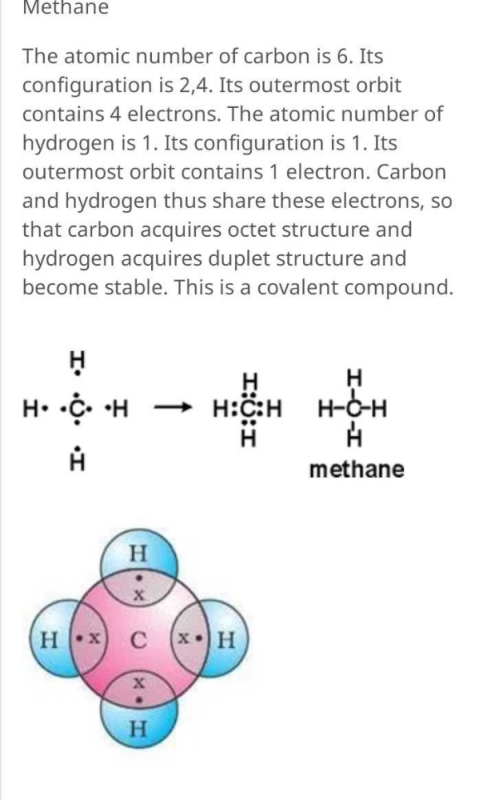
Draw the electron dot diagram of a methane molecule [C=6,H=1 ...
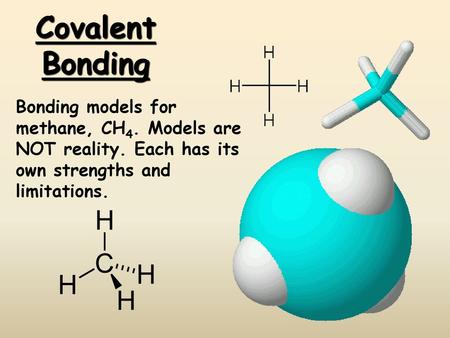
Covalent Bonding Bonding models for methane, CH4. Models are NOT reality. Each has its own strengths and limitations.

Structure of methane, CH4. The carbon atom is shown in grey ...

PPT - Lewis Dot Structure PowerPoint Presentation, free ...

Draw an electron dot diagram to show the formation of each of ...
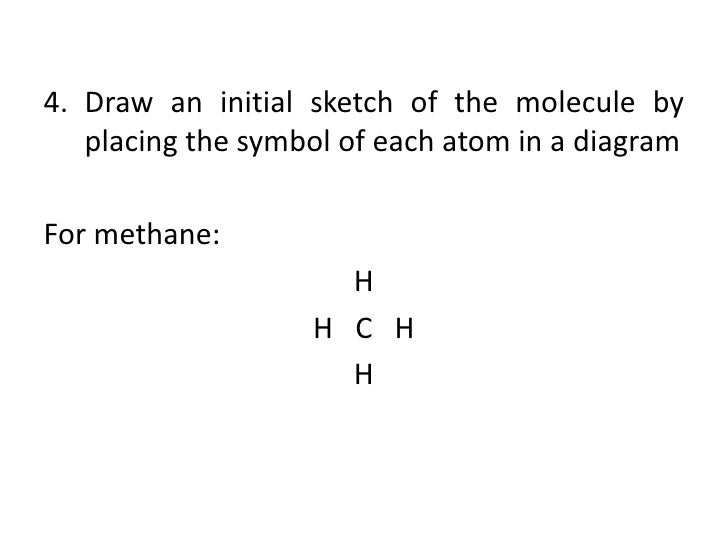
How to draw methane ch4 lewis structure

4.8: Polyatomic Molecules- Water, Ammonia, and Methane ...

How to determine the Lewis structure for CH4 - Quora

explain the bonding in Methane molecule using electron dot ...
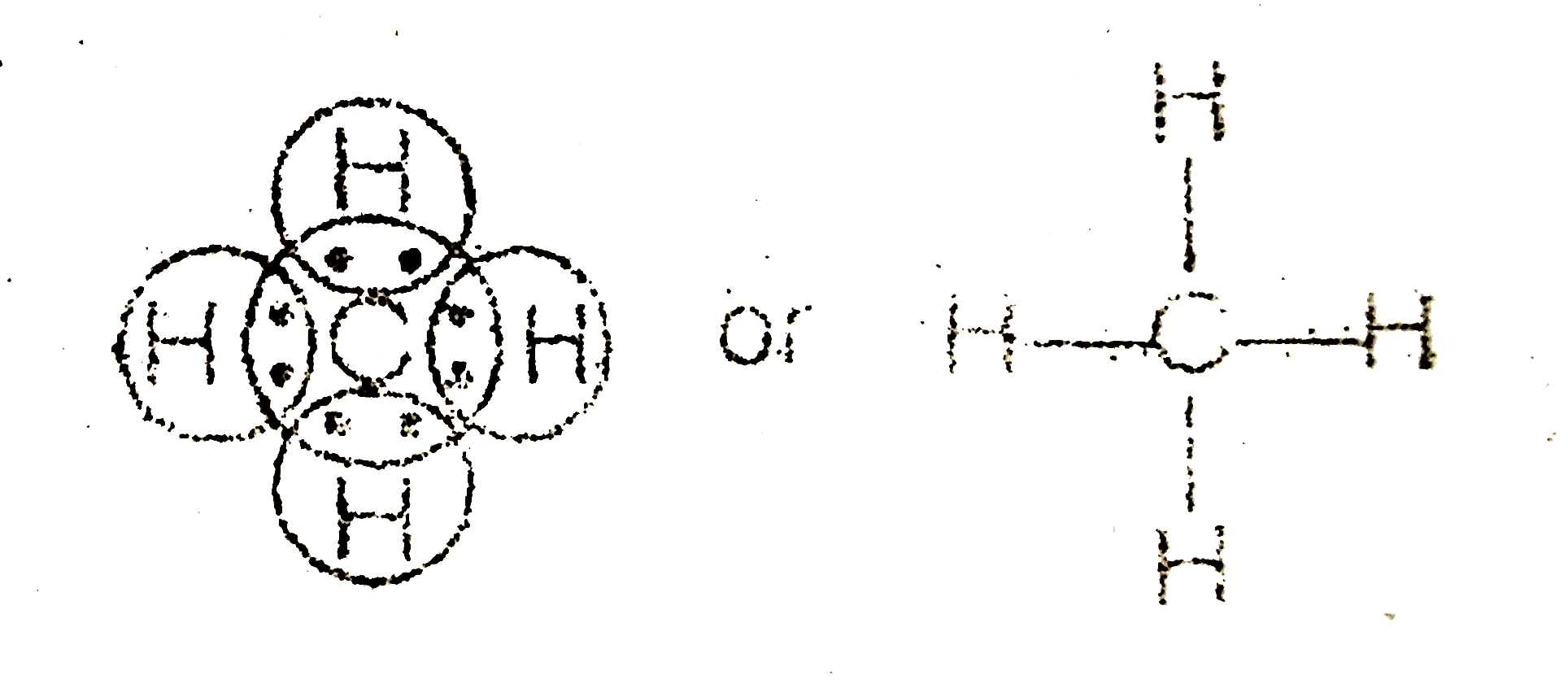
Draw the Lewis dot structure of Methane (CH_(4)) molecule .

Difference Between Lewis Dot Symbol and Lewis Structure ...

Lewis Dot Symbols and Lewis Structures | Boundless Chemistry
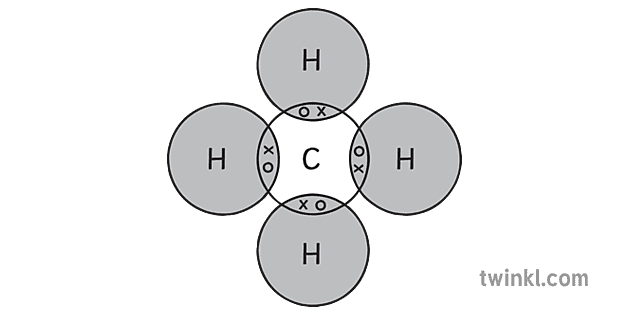
Ch4 Methane Covalent Bonding Dot Cross Diagram Science ...

electron-dot structure and line structure of methane molecule ...

methane molecule CH4 Lewis dot & cross electronic diagram ...
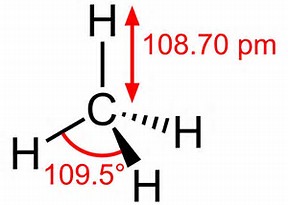
In the three-dimensional structure of methane, CH4, where are ...

What is methane? Draw its electron dot structure. | Snapsolve

How to determine the Lewis dot structure for methane - Quora
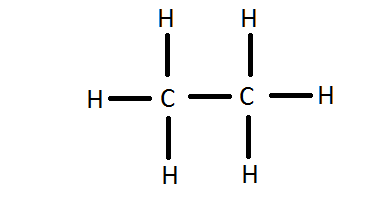
How can I write the Lewis dot structure for C2H6? | Socratic

Lewis Dot Structures - Understanding Molecular Structure

























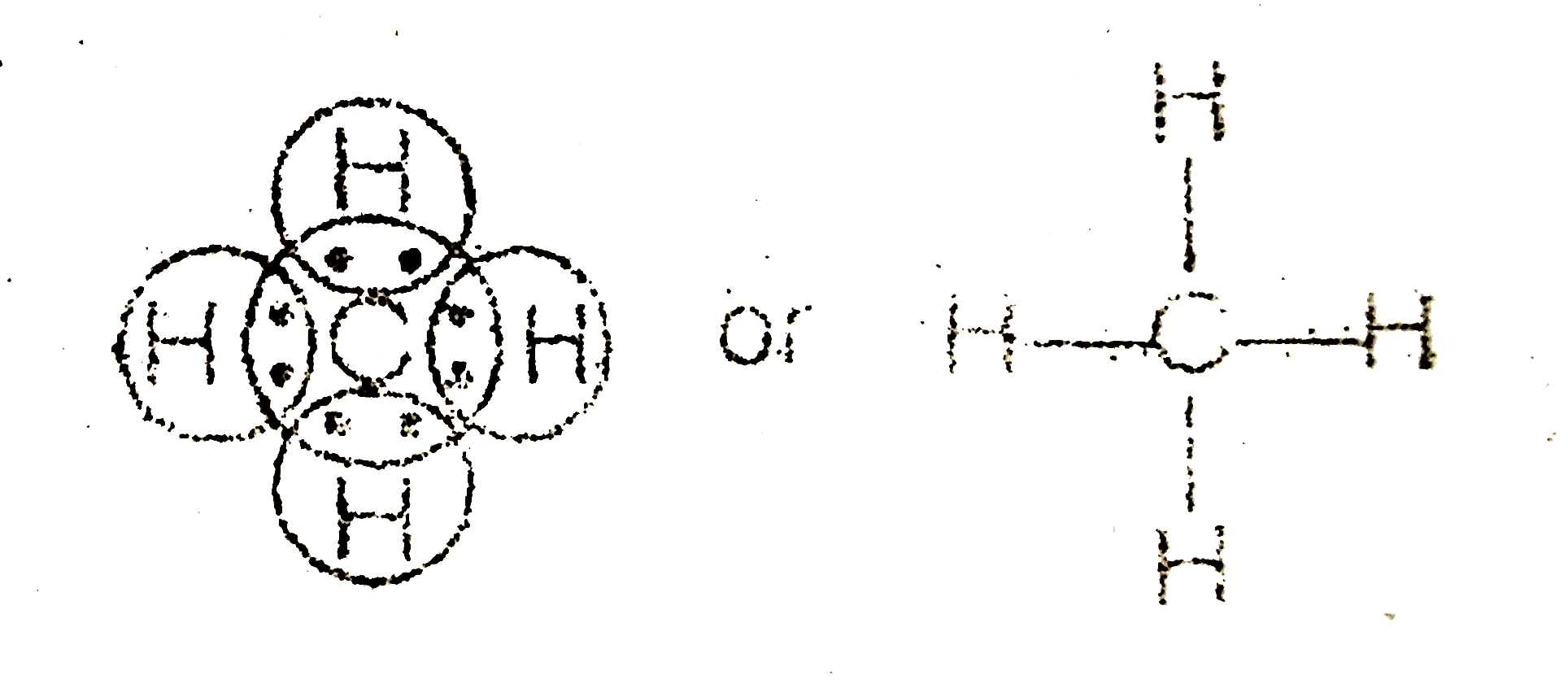








0 Response to "39 dot diagram for ch4"
Post a Comment