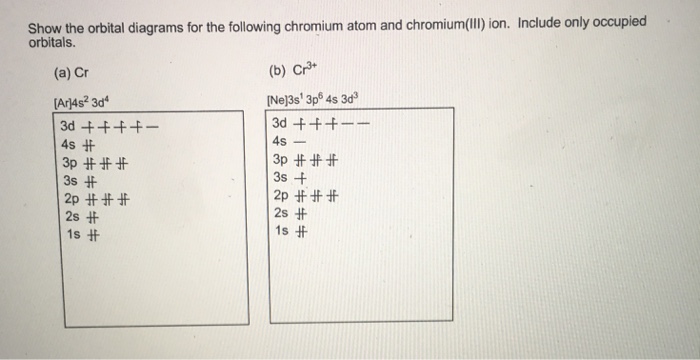
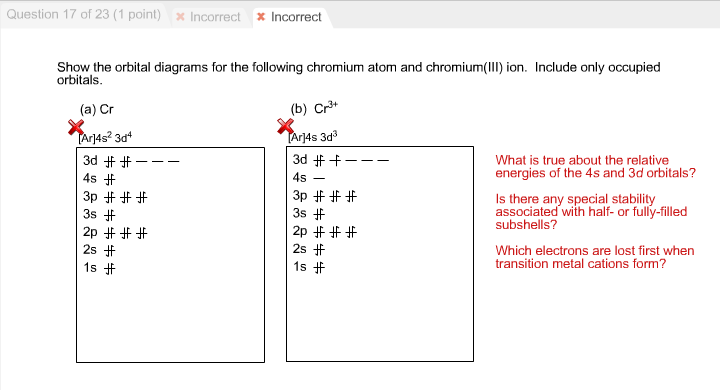
38 orbital diagram for cr3+
Orbital Diagram For V5+ - schematron.org Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic. Orbital Diagram For Strontium - schematron.org In an orbital (box) diagram a box represents each notation and an orbital diagram. strontium atom (a) in the spdf notation and (b) in the. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of strontium (atomic number: 38), the most.
Answered: Write orbital diagrams for each ion and… | bartleby Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. V5 + b. Cr3 + c. Ni2 + d. Fe3 +. Expert Solution.

Orbital diagram for cr3+
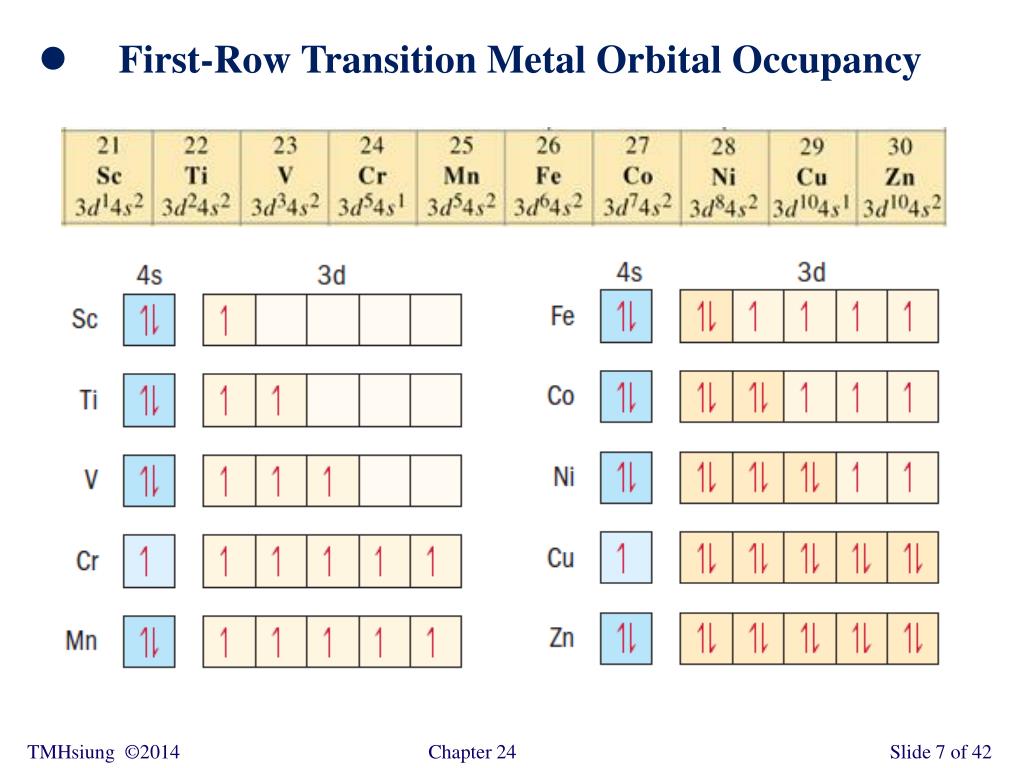
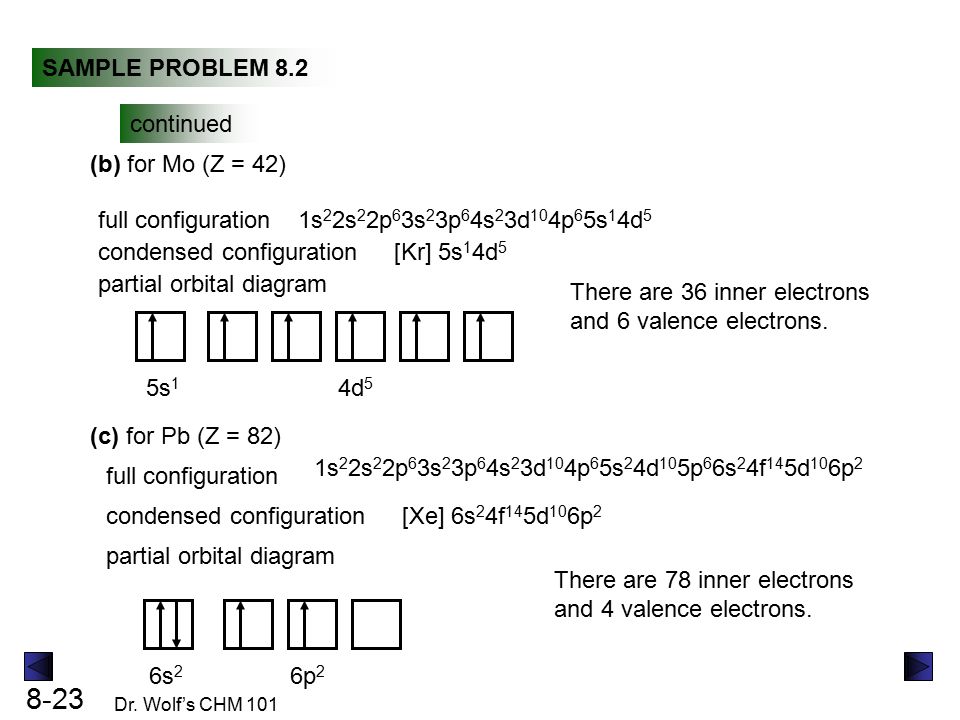
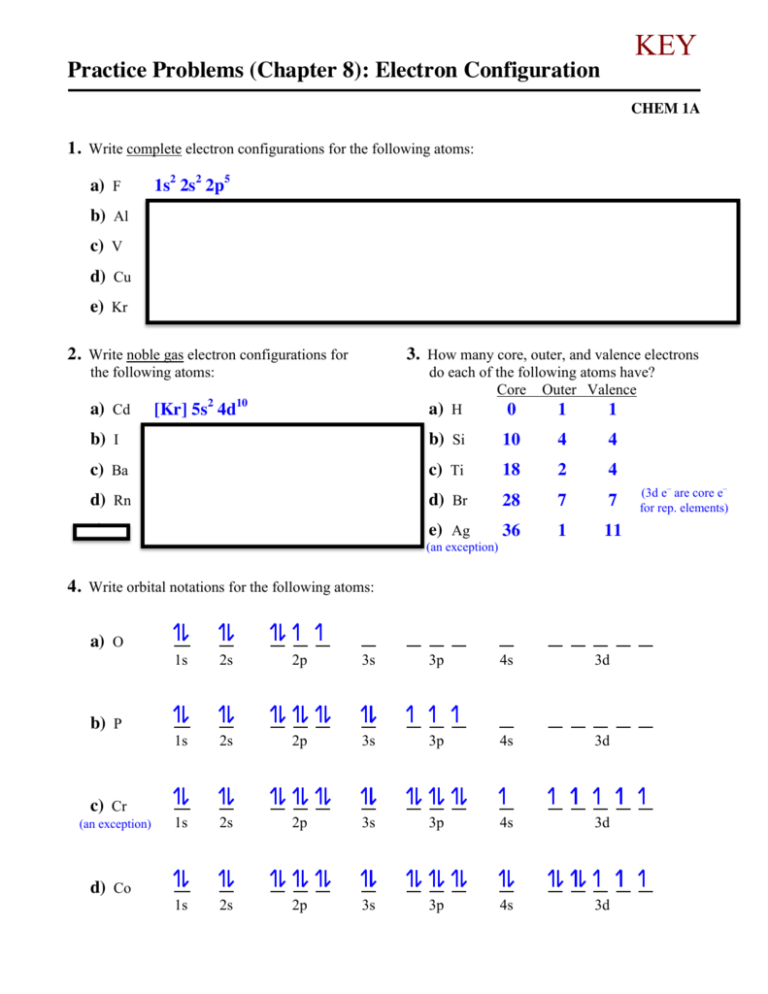
Electron Configuration for Chromium (Cr, Cr2+, Cr3+) After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s. Chapter 7: Quantum Theory and the Electronic Structure of ... The orbital diagram for a ground state carbon atom is. 15. Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these ... Write the ground-state electron configuration for Cr3+. 94. Write the ground-state electron configuration for Ni2 ... Problem Set #4 (Ch 3, 4) Flashcards - Quizlet Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+ Determine if the ion is diamagnetic or paramagnetic. [Ne] 3s^2 3p^6 [Ar] 4s^0 3d^3 [Ar] 4s^0 3d^8 [Ar] 4s^0 3d^5 diamagnetic: V5+ paramagnetic: Cr3+, Ni2+, Fe+. Choose the larger atom from each of the following pairs. Al or In Br or Ar S or Sn Si or Cl. In Br Sn Si. Choose the ...
Orbital diagram for cr3+. Solved Part B Enter an orbital diagram for Cr3+. Drag the ... Best Answer. This is the best answer based on feedback and ratings. Transcribed image text: Part B Enter an orbital diagram for Cr3+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Targets may be left blank, such as for unused orbitals. Reset Help 1111s 2s 4s 2p 30 40 30 G1 G1 G1 ... Bromine Orbital Diagram - Wiring Diagrams Bromine Orbital Diagram. Answer to Write the electron configuration and give the orbital diagram of a bromine (Br) atom (Z = 35). the σ bonds. I've drawn the overlaps below in the MO diagrams. Each bromine would donate one 4pz electron to form a σ -bonding orbital. Answer to Draw an orbital diagram for each element: (a) magnesium; (b ... Write orbital diagrams for each ion and indicate whether ... Step 1. 1 of 6. In writing orbital diagrams, first, determine the electron configuration of the neutral atom and remove electrons accordingly. Diamagnetic ions contain no unpaired electrons. Paramagnetic ions have unpaired electrons. Determine the orbital diagrams of a. V. 5 + ^ {5+} 5 +. b. PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals?
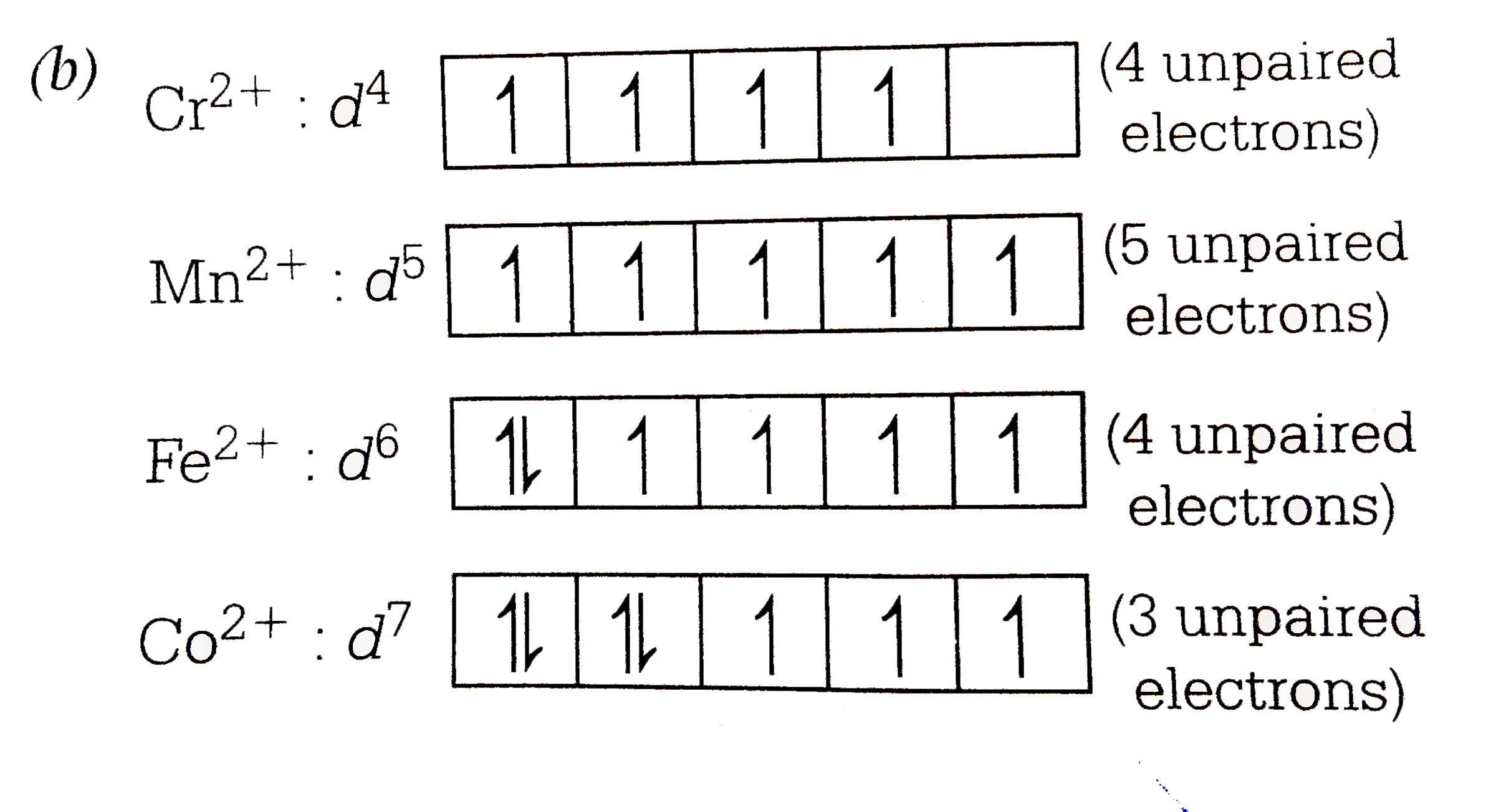
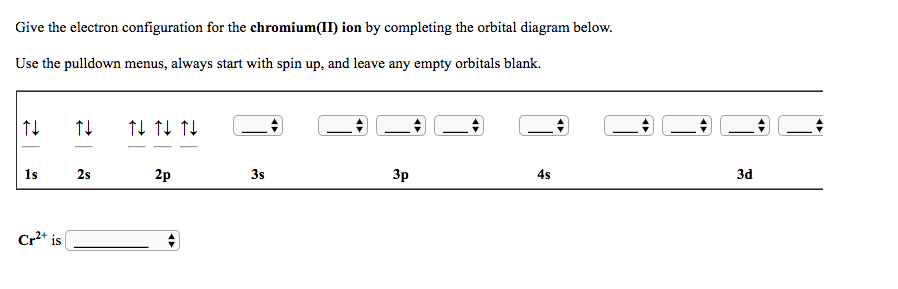
Chromium(Cr) electron configuration and orbital diagram Atomic Orbital Diagram for Chromium (Cr) Chromium ion (Cr 2+, Cr 3+) electron configuration Ground state electron configuration of chromium (Cr) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 1. The electron configuration shows that the last shell of chromium has an electron and the d-orbital has a total of five electrons. PDF Electron configuration of cr3 Electron configuration of cr3 ... Why are unique external electrons included in the orbital filling diagram? They are the only ones involved in chemical reactions and bonding. 2s Orbital is farther from the nucleus meaning that has more energy. Beginner enthalpy ionization. Entertainment ionization of elements is the quantity of energy that an ... Orbital Diagram For Fe3+ Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms. SOLVED:Write orbital diagrams for each ion and determine ... Answer. Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. V5+ b. Cr3+ c. Ni2+ d. Fe3+
V5+ Orbital Diagram - schematron.org Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V. (1) Question: What is the maximum number of electrons each orbital (s, p, d, f) can transition metals with their common oxidation states: A) V2+. B) V3+. C) V5+. This is what I believe to be the orbital diagram of ... Mo3+ Orbital Diagram - Wiring Diagrams However, even though the 5s orbital is lower in energy than the 4d orbital, the electrons in the 4d orbitals shield the electron in the 5s orbitals. can be accommodated in the metal d orbitals. • d0 ions d3 ions - V2+, Ta2+, Cr3+, Mo3+, Mn4+, etc. . σ-ML4 Tetrahedral MO Diagram e. Answer to Write orbital diagram for Mo3+. SOLVED:Draw the octahedral crystal field splitting diagram ... Ordeal Atrous left. So that's how Hey hi. Spin crystal filled orbital spitting diagram for em in Tripolis looks like and then for if it topless we has we have ah ah formal alike this hand in argon and then three d six so for this case, we have to find out the low spin or without spitting. So this is a law spin. Solved Part A Enter an orbital diagram for V5+ Drag the ... Transcribed image text: Part A Enter an orbital diagram for V5+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled Reset Help P 111 1s 25 38 4s 2p 3p 4p 3d 61 G1 G1 GIG1 G1 GIG1 G1 G1 G1G1G1 G1|| G1 G16161 62 G2 G2 G2 G2 G2 G2 G2 Submit Request Answer Part B Enter an orbital diagram for Cr3 Drag the ...
PDF Orbital diagram for zinc The 1s orbital at the bottom of the diagram is orbital with electrons of the lowest energy. Zinc (Zn). So the only possible stable configuration would be 4s2 3d3. Fill in the orbital energy diagram for zinc ion The lowest E levels are already filled in for you . Write an orbital diagram for the state of the earth of the zinc atom.
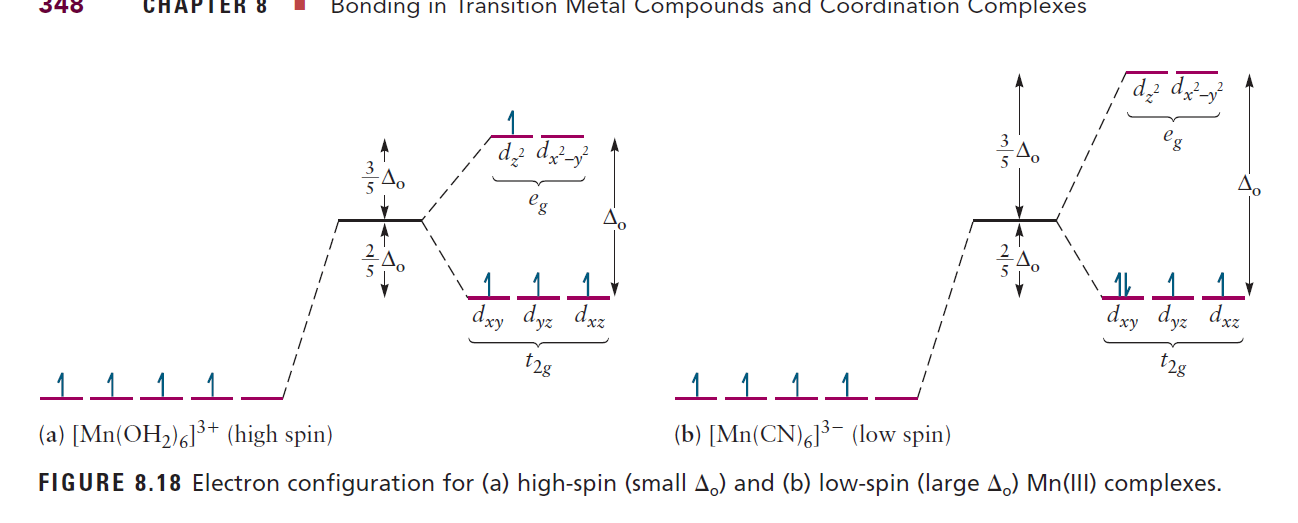
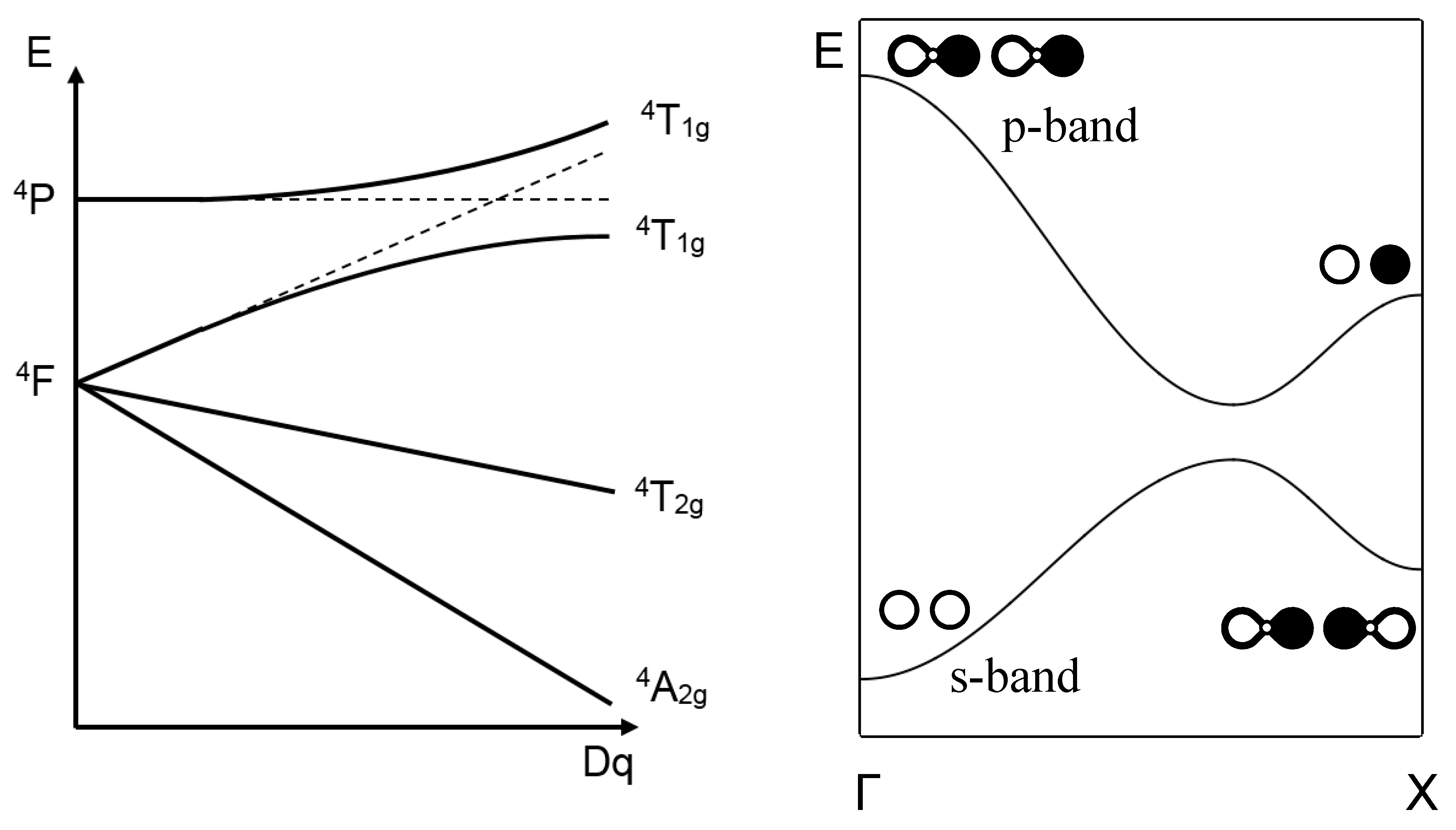
PDF Principles of Chemical Science, Solutions for Lecture 28 ... an crystal field splitting diagrams to show orbital occupancies in both weak and strong octahedral fields, and (ii) indicate the number of unpaired electrons in each case. Label . the diagrams (iii) weak or strong field, (iv) high spin or low spin (as appropriate), (v) with the names of the d-orbitals, and (vi) with the appropriate orbital sets ...
Orbital Diagram of All Elements (Diagrams given Inside) Apr 10, 2021 · Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
Orbital Diagram For Au+ - schematron.org The atomic number of Au is Therefore, its For Au+, one electron is removed from the outermost 6s orbital, making the configuration. Electron Configuration, [Xe] 4f14 5d10 6s1. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. Orbital Diagram. 1s. ↿⇂. 2s. ↿⇂. 2p. Write orbital diagram for Au+? ↿⇂. ↿⇂. ↿⇂.
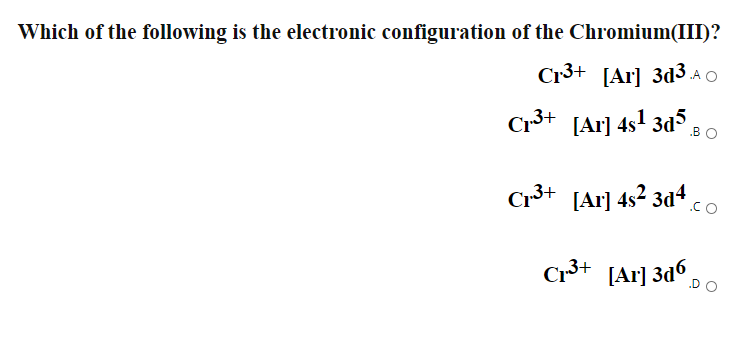
What is the electron configuration of Cr 3+? | Socratic Nov 30, 2015 · Cr:1s22s22p63s23p64s13d5. Note that it is 4s13d5 and not 4s23d4 because a half filled d orbital is more stable than a partially filled d orbital. However, the chromium ion Cr3+ possesses 24e− −3e− = 21e− due to the loss of 3 of its electrons. Thus, the electron configuration of Cr3+ is: Cr3+:1s22s22p63s23p64s03d3.
Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to ... To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number...
Spectrochemical Series for Cobalt (III) - Texas A&M University 3. 3+Using your answer from Question 2, draw a d-orbital splitting diagram for low-spin Co similar to the one given for Cr3+ 3+in the introduction to this experiment. Almost all Co complexes are low-spin, with a minimum number of unpaired electrons. (Refer to your text for further explanation.) 4.
The electronic configuration of Cr having atomic number of 24 is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5 which is half-filled d-orbital. Cr3+ has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [Ar]3d3.
SOLVED:Write orbital diagrams for each ion and determine ... take a look here at utilizing the periodic table too. Do some orbital configurations or diagrams for some metal cat ions. So first example is going to be for chromium three plus. So as we follow the periodic table and fill the electrons properly, first row of the periodic table. Um we feel the first two electrons in the structure in the one S sub shell with its one orbital.
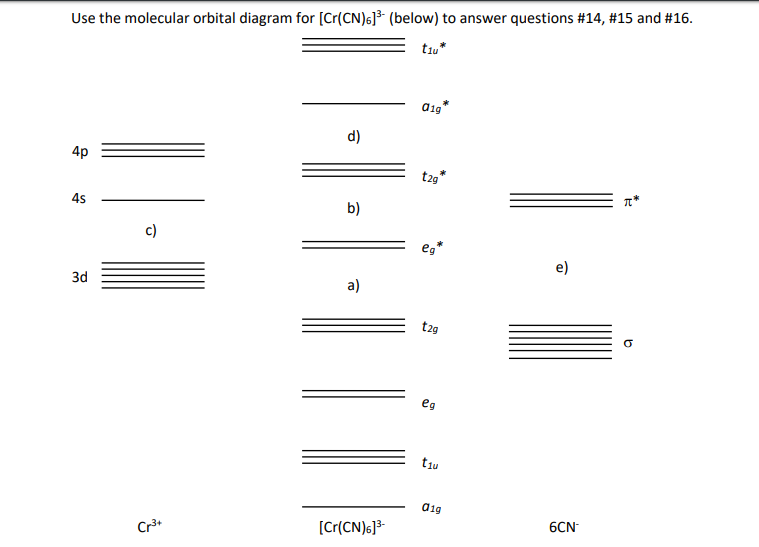
PDF MO Diagrams for More Complex Molecules • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
Problem Set #4 (Ch 3, 4) Flashcards - Quizlet Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+ Determine if the ion is diamagnetic or paramagnetic. [Ne] 3s^2 3p^6 [Ar] 4s^0 3d^3 [Ar] 4s^0 3d^8 [Ar] 4s^0 3d^5 diamagnetic: V5+ paramagnetic: Cr3+, Ni2+, Fe+. Choose the larger atom from each of the following pairs. Al or In Br or Ar S or Sn Si or Cl. In Br Sn Si. Choose the ...
Chapter 7: Quantum Theory and the Electronic Structure of ... The orbital diagram for a ground state carbon atom is. 15. Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these ... Write the ground-state electron configuration for Cr3+. 94. Write the ground-state electron configuration for Ni2 ...
Electron Configuration for Chromium (Cr, Cr2+, Cr3+) After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s.
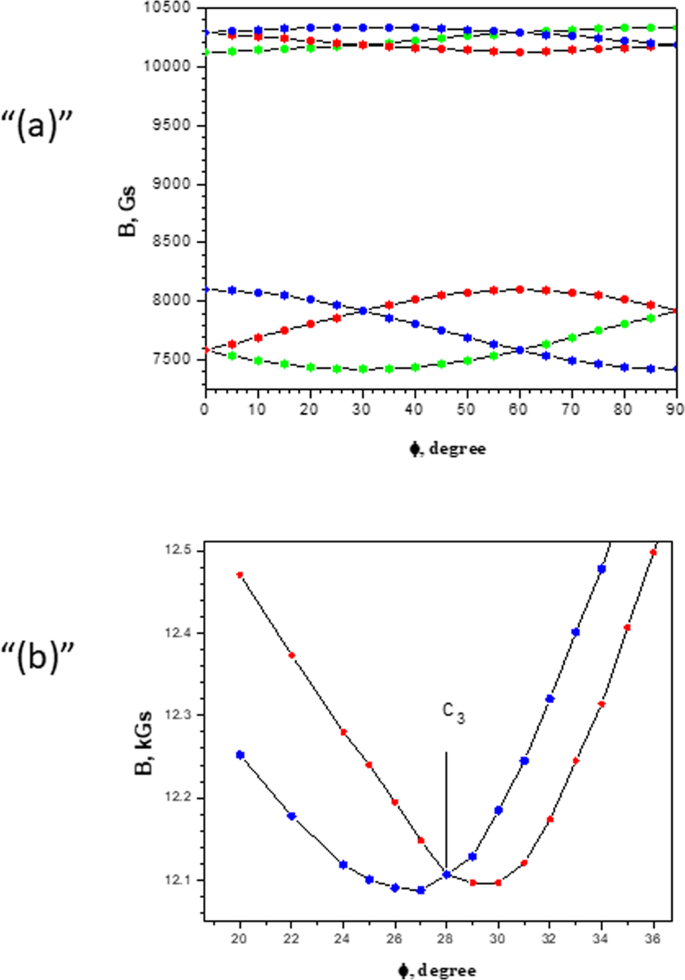

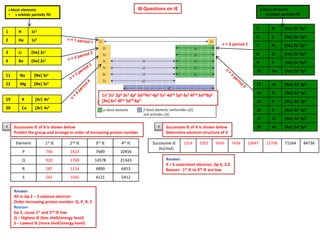






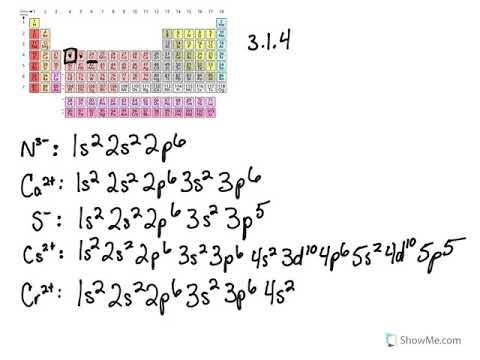



0 Response to "38 orbital diagram for cr3+"
Post a Comment