36 magnesium electron dot diagram
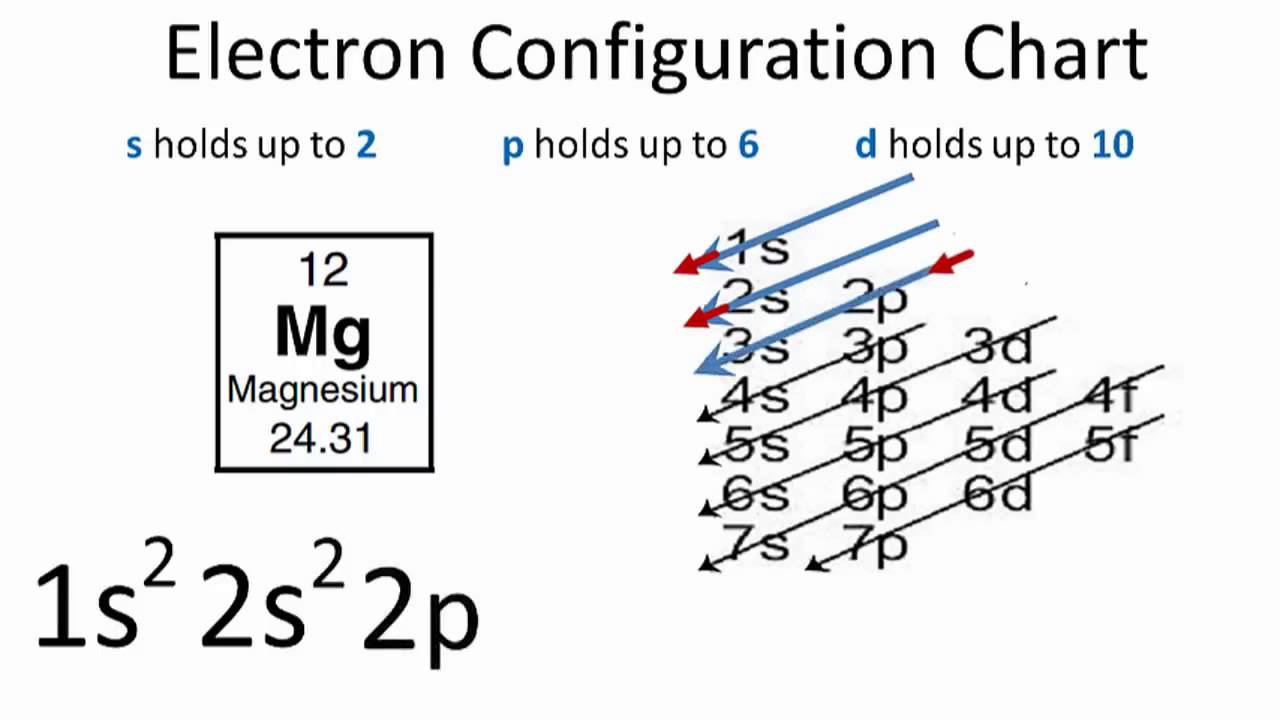
Lewis acids and bases - Wikipedia A center dot may also be used to represent a Lewis adduct, such as Me 3 B·NH 3. Another example is boron trifluoride diethyl etherate , BF 3 ·Et 2 O . In a slightly different usage, the center dot is also used to represent hydrate coordination in various crystals, as in MgSO 4 ·7H 2 O for hydrated magnesium sulfate , irrespective of whether the water forms a dative bond with the … Write the electron dot structure for magnesium and class ... First, we have to draw the electron dot structure of magnesium and chlorine, to draw electron dot structure we have to write the electron configuration of magnesium and chlorine. Magnesium has atomic number 12, so there are 12 electrons in magnesium atom. Its electronic configuration will be: 1 s 2 2 s 2 2 p 6 3 s 2
How di I write the Lewis structure of magnesium chloride ... "Cl" can get a noble gas s^2p^6 configuration by gaining an electron and forming a chloride ion, "Cl"^"-". The Lewis structure of "MgCl"_2 is therefore (From ) The compound is held together by electrostatic attractions between the positively charged magnesium ion and the negatively charged chloride ions.

Magnesium electron dot diagram
1.2.2 Structure and Bonding Revision (ii) Draw a ‘dot-and-cross’ diagram to show the bonding in magnesium sulfide. Show outer electron shells only. [2] [Total 3 marks] 2. ‘Dot-and-cross’ diagrams can be used to predict the shape of covalent molecules. Fluorine has a covalent oxide called difluorine oxide, F2O. The oxygen atom is covalently bonded to each fluorine atom. (i ... How do you draw a Magnesium electron dot diagram? - Answers What is the electron dot diagram for magnesium nitride? Magnesium Nitride is Mg3N2. What I think you do is draw it Mg N Mg N Mg and then draw 8 electrons around each Nitrogen so that Mg shares its ... Electron Dot Diagrams and Lewis Structures Flashcards ... electron dot diagram for Phosphorus. Lewis structure for PCl₃. Lewis structure for CH₄. Lewis structure for CH₃Br. Lewis structure for F₂O. Lewis structure for IBr. 6 dots around I, single bond, 6 dots around Br. Lewis structure for NH₂Cl. N in middle, 2 dots around N, single bonded to H on both left and right, single bonded down with ...
Magnesium electron dot diagram. What is the electron dot diagram for magnesium oxide ... Elemental magnesium has 12 nuclear protons, Z = 12. It has 2 valence electrons that are conceived to be lost when it undergoes oxidation to M g2+. M g → M g2+ + 2e− (i) Elemental (atomic!) oxygen has 8 electrons, Z = 8. The oxide anion thus has 10 electrons upon reduction: O +2e− → O2− (ii) So (i) +(ii) = M g(s) + 1 2O2(g) → M gO(s) Answer link Magnesium Fluoride Lewis Dot Diagram Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluoride?. MgF2 (2 should be subscript) because fluoride is -1 electron and Mg is 2+ d) draw the dot diagram for magnesium fluoride. since this is an. What is the Lewis dot diagram for magnesium? - Quora Step-1:Mg Valence Electrons To draw the lewis Dot structure of magnesium (Mg) , we have to find out the valence electrons of magnesium (Mg) first.We express valence electrons as dots in lewis dot structure. To get the valence electrons of magnesium (Mg) ,we need to look at the electronic configuration of magnesium (Mg). Mg (12)=1s²2s²2p⁶3s² MgO Lewis Structure, Molecular Structure, Bonding, and ... Steps to Draw the Lewis Structure of magnesium oxide (MgO) Step 1. Look for how many valence electrons already available in one magnesium oxide molecule: It is eight as two are coming from the Magnesium atom and six are coming from the Oxygen atom. Step 2.
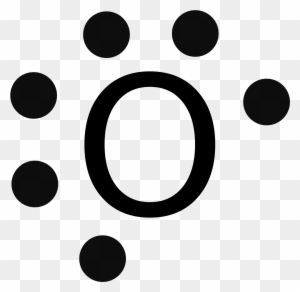
What is the electron dot diagram of magnesium oxid class ... Magnesium oxide consists of magnesium and oxygen and has the formula MgO, which consist of ionic bonds. Complete answer: Electron dot diagram or structure is the diagram that represents the electrons of an atom, that are present in the valence shell in the form of dots. These dots are made around the symbol of the atom. COMBINED SCIENCE: TRILOGY - AQA Write about electron transfer in your answer. [4 marks] 6 *06* IB/M/Jun18/8464/C/1H Do not write outside the box 8 0 2 ... Complete the dot and cross diagram on Figure 4 You should show only the electrons in the outer shells. [2 marks] Figure 4 . ... Explain which species is reduced in the reaction between magnesium and iron chloride. 3 Mg + 2 ... Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ... Magnesium phosphide | Mg3P2 - PubChem Magnesium phosphide is a white crystalline solid. It reacts violently with water and may ignite upon contact with air. It is toxic by ingestion. It is used to make other chemicals. CAMEO Chemicals YELLOW-TO-GREEN CRYSTALS. ILO International Chemical Safety Cards (ICSC) 3.2.2 Color/Form Yellow cubic crystals
PDF Electron dot diagram of magnesium oxide Electron dot diagram of magnesium oxide Solution: First examine the electron arrangement of the magnesium and nitrogen atoms. Symbol Atomic No. Bohr diagram Group No. Lewis Dots Mg 12 2 - 8 - 2 2 2 N 7 2 - 5 5 5 Write the Lewis symbols for each atom. See graphic on the left. Cambridge O Level 6 UCLES 2021 5070/21/M/J/21 3 Petroleum (crude oil) is a mixture of hydrocarbons. (a) Petroleum (crude oil) is separated into fractions such as liquefied petroleum gas, petrol (gasoline) and naphtha. (i) Name the process used to separate petroleum (crude oil) into fractions. [1] (ii) Name one other fraction separated from petroleum (crude oil). Give a large-scale use for this … Octet rule - Wikipedia The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium or magnesium. Which diagram is the correct electron dot diagram for ... Magnesium is the element of second group and third period. The electronic configuration of magnesium is - 2, 8, 2 or. There are 2 valence electrons of magnesium. Only the valence electrons are shown by dots in the Lewis structure. As, stated above, there are only two valence electrons of magnesium, so in the Lewis structure, two dots are made ...
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Give Electron Dot Diagram of the Following: Magnesium ... Give Electron Dot Diagram of the Following: Magnesium Chloride . CISCE ICSE Class 10. Question Papers 359 ... Question Bank Solutions 24872. Concept Notes & Videos 608. Time Tables 16. Syllabus. Advertisement Remove all ads. Give Electron Dot Diagram of the Following: Magnesium Chloride - Chemistry. Advertisement Remove all ads. Advertisement ...
Draw the Lewis Structure of MgCl2 (magnesium chloride ... One magnesium atom loses two electrons, to become a +2 ion (cation).Two chlorine atoms gain one electron each to become two -1 ions (anions).These are held t...
MgF2 Lewis Structure, Geometry, Hybridization, and ... The atomic number of magnesium is 12 where its electronic configuration is 1s2 2s2 2p6 3s2. This makes the total number of valence electrons in magnesium 2. Whereas for fluorine the atomic number is 9 and its electronic configuration is 1s2 2s2 2p5. Here, the valence electrons in fluorine are 9.
Magnesium Bohr Model - How to draw Bohr diagram for ... Bohr's diagram of Magnesium has three electron shells (K, L, and M), the inner shell is the K-shell and the outermost shell is M-shell. Hence, the electrons found in the M-shell of the Magnesium atom are its valence electrons because it is the outermost shell also called the valence shell.
PDF Magnesium iodine lewis dot structure Bookmark the lewis dot diagram for magnesium lewis structure lewis dot systems. For mgo we have an ionic compound and we need to take that into account when we draw the lewis structure. No templates or rules required. Lewis electron dot diagrams. Magnesium iodine lewis dot structure
Draw the Electron Dot Diagram and Structure of : Magnesium ... Draw the Electron Dot Diagram and Structure of : Magnesium Chloride . CISCE ICSE Class 10. Question Papers 359. ... Question Bank Solutions 24781. Concept Notes & Videos 608. Time Tables 16. Syllabus. Advertisement Remove all ads. Draw the Electron Dot Diagram and Structure of : Magnesium Chloride - Chemistry. Advertisement Remove all ads.
Draw Electron Dot Structure For The Formation Of Magnesium ... Draw Electron Dot Structure For The Formation Of Magnesium Oxide Magnesium Oxide (MgO) is the most chemically active element. Its atomic number is 12, placed in 2nd group 2 and 3rd period in the modern periodic table. Formation Magnesium oxide (MgO) by the transfer of electrons
Lewis Dot Structure for Magnesium (Mg) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for Mg (Magnesium). I show you where Magnesium is on the periodic table and how to determi...
Magnesium hydroxide | H2MgO2 - PubChem Magnesium hydroxide (JAN/USP) Magnesium hydroxide [USP:JAN] CS-B1709 Magnesium hydroxide, BioXtra, >=95% Magnesium hydroxide, reagent grade, 95% Magnesium hydroxide, 95.00-100.50% Magnesium hydroxide, Vetec (TM) reagent grade D00731 EC 215-170-3 Magnesium hydroxide, BioUltra, >=99.0% (KT) Magnesium hydroxide, SAJ first grade, >=95.0%
Lewis Dot Diagram For Magnesium Fluoride - schematron.org which lewis electron-dot diagram represents an atom in the ground state for a the correct lewis electron-dot structure for the compound magnesium fluoride?.dec 18, · best answer: magnesium has 2 valence electrons and fluorine has 7 valence electrons in order for the 2 elements to combine, you need 1 mg atom and 2 f atoms the mg gives one …
The following shows the electronic arrangement of two ... LLLL 4. Write the electron dot structure for! magnesium and chlorine. Show the formation of magnesium chloride by the transfer of I electrons.
Magnesium Fluoride Lewis Dot Diagram - schematron.org the bond order, determined by the lewis structure, is the number of pairs of.dec 18, · best answer: magnesium has 2 valence electrons and fluorine has 7 valence electrons in order for the 2 elements to combine, you need 1 mg atom and 2 f atoms the mg gives one electrons to each of the f atoms, allowing the mg to lose its 2 valence electrons and …
Lewis Electron Dot Diagrams - Welcome to CK-12 Foundation Explain the meaning of an electron dot diagram. Draw electron dot diagrams for given elements. ... Look at the electron configuration for magnesium. Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for in a box with all of its core electrons (i.e., ). Then we place the valence electrons around ...
Electron Dot Diagrams and Lewis Structures Flashcards ... electron dot diagram for Phosphorus. Lewis structure for PCl₃. Lewis structure for CH₄. Lewis structure for CH₃Br. Lewis structure for F₂O. Lewis structure for IBr. 6 dots around I, single bond, 6 dots around Br. Lewis structure for NH₂Cl. N in middle, 2 dots around N, single bonded to H on both left and right, single bonded down with ...
How do you draw a Magnesium electron dot diagram? - Answers What is the electron dot diagram for magnesium nitride? Magnesium Nitride is Mg3N2. What I think you do is draw it Mg N Mg N Mg and then draw 8 electrons around each Nitrogen so that Mg shares its ...









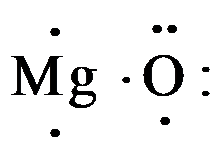
0 Response to "36 magnesium electron dot diagram"
Post a Comment