37 ethene molecular orbital diagram
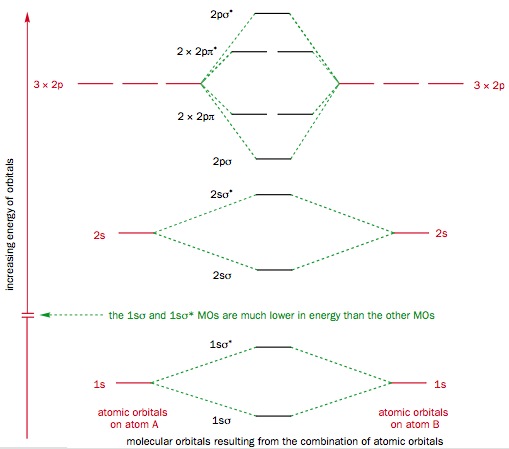
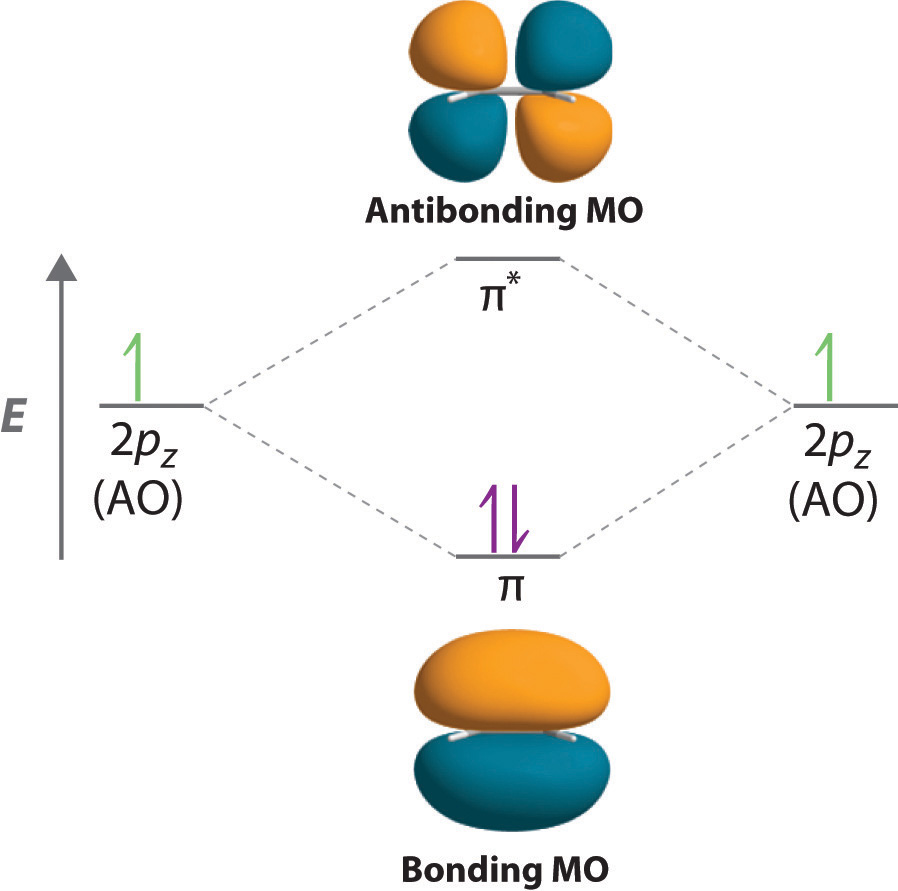
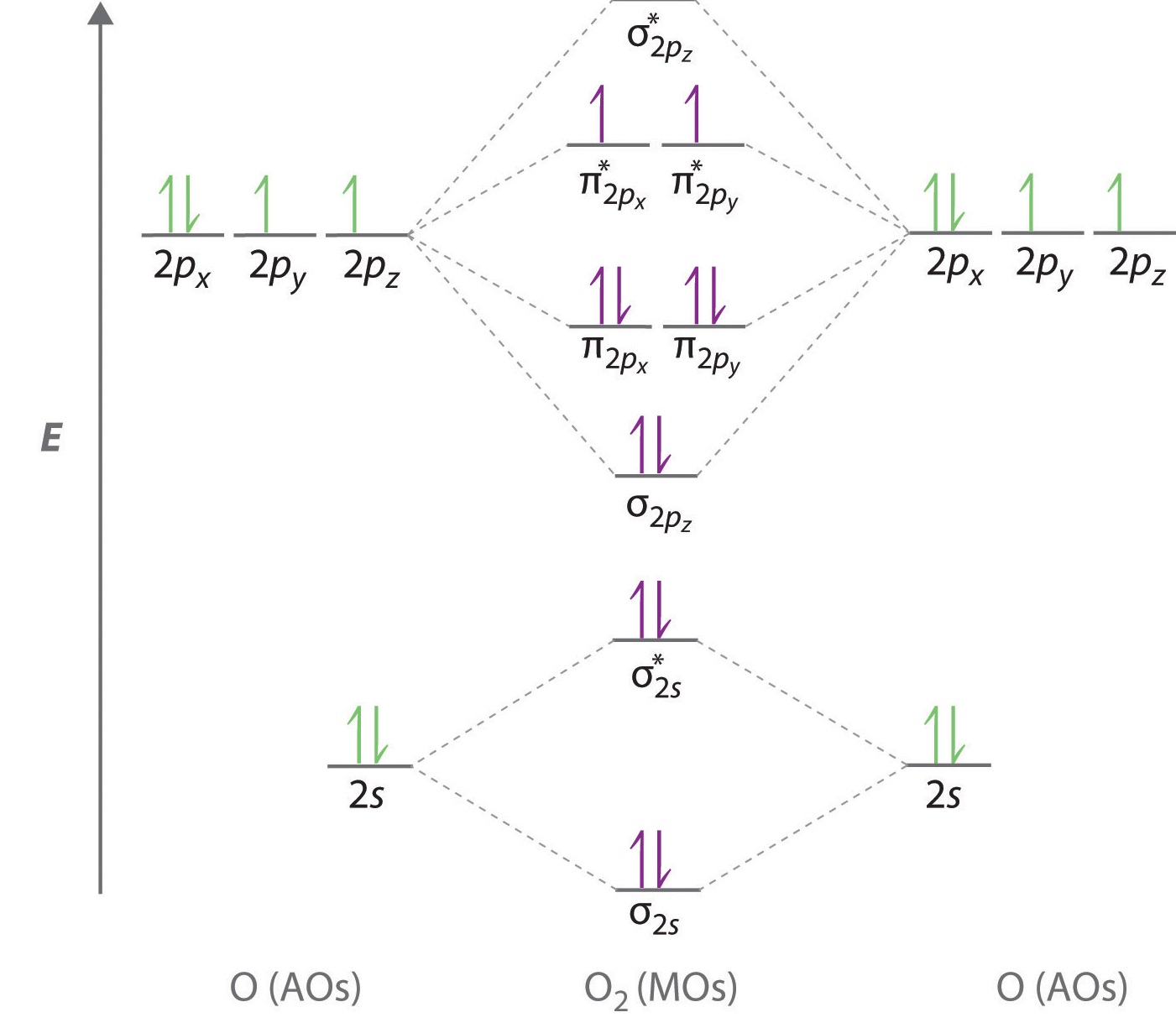
Chapter 1: Molecular Orbital Concepts Consider the pi bond of ethene in simple molecular orbital terms (The qualitative results would be the same for any pi or sigma bond. q The overlap of the two atomic orbitals (AO's) results in the formation of two molecular orbitals (MO's), one of which is lower in energy than the original AO's (the bonding MO or BMO) and the other higher ... PD Dr. Stefan Immel - Molecular Orbitals - Ethene Below on the right, the schematic drawing indicates the major contributions of atomic orbitals (AOs) to the molecular orbitals (MOs) of ethene. With increasing energy of the orbitals (from bottom to top), the number of nodal planes (not necessarily real "planar" planes) increases and the symmetry decreases.
Molecular Orbital theory: Ethane • In this manner, ethane can be constructed from MO's of two pyramidal CH3 groups. • Only consider the first-order mixings: • σ (CH3) and π (CH3) orbitals are primarily C-H bonding - do not change much with mixing in forming C-C bond. • σ (out) is directed away from hydrogens and towards the C-C bond

Ethene molecular orbital diagram
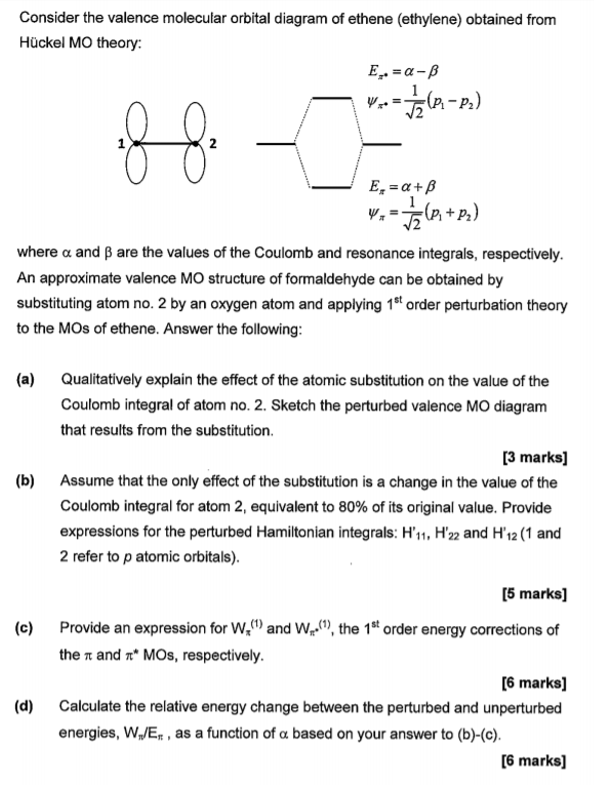
Solved a) Considering the molecular orbital diagram for ... a) Considering the molecular orbital diagram for ethylene above, draw the molecules (with some kind of three-dimensional representation) of the initial interaction of ethylene with a very strong acid (represent this as H-A). b) Finish the molecular orbital diagram of formaldehyde (H 2 CO) by making the molecular orbitals in the middle. Don't ... Molecular orbitals - Triple bonds, Ethyne Molecular orbitals - Double bonds, Ethene. Electronic configuration of atoms (Part 1) Next Top. ABPI. The Association of the British Pharmaceutical. Industry is a company limited by guarantee registered. in England and Wales (registered number 09826787) 7th Floor Southside, ... 13.2. Molecular orbitals for ethene | Organic Chemistry II Molecular orbitals for ethene (ethylene) In the bonding pi orbital, the two shaded lobes of the p orbitals interact constructively with each other, as do the two unshaded lobes (remember, the arbitrary shading choice represents mathematical (+) and (-) signs for the mathematical wavefunction describing the orbital).
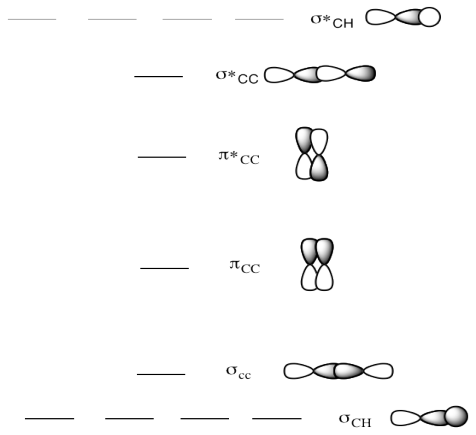
Ethene molecular orbital diagram. PDF ORBITALS and MOLECULAR REPRESENTATION For this we need to picture atomic and molecular orbitals. l = 0 2 ATOMIC ORBITALS 2p x 2p y 2p z l = 1 x y z n = 2 This is an accurate representation of a 2p x orbital. This is a common picture of a p x orbital This simplifi ed p x orbital is often useful. A hand drawn version does not have to be exact. Use this box to draw a p z orbital. Solved Draw the molecular orbital diagram for ethene. Show ... Question: Draw the molecular orbital diagram for ethene. Show the interaction of the "p" orbitals that would result in the * (antibonding) molecular orbital. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Consider ethylene (also called ethene) - MSU chemistry • Construct an MO diagram for the C-C σ-bond in ethane (C 2H 6). Forming π-bonds From "Leftover" pOrbitals • Construct an MO diagram for the C=C "-bond in ethene (H2C=CH2). • Draw a complete MO diagram for all the bonds in ethene. What can we say, at this point, about the relative energy levels of the orbitals in this molecule PDF Molecular Orbitals: Example 1: Ethylene - ualberta.ca Molecular Orbitals: Example 1: Ethylene E E H2CCH2 p 2 sp2 1s p 2 sp2 1s σ σ* π π* A.O. A.O. C C HHC C C from other end of double bond A.O. means atomic orbitals (s, sp2, p) M.O. means molecular orbitals (σ , π ) C from left end of double bond M.O. Looking at both sigma and pi bonds
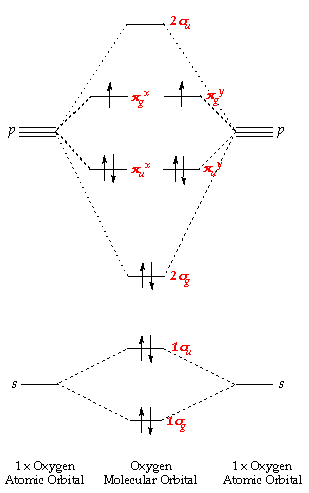
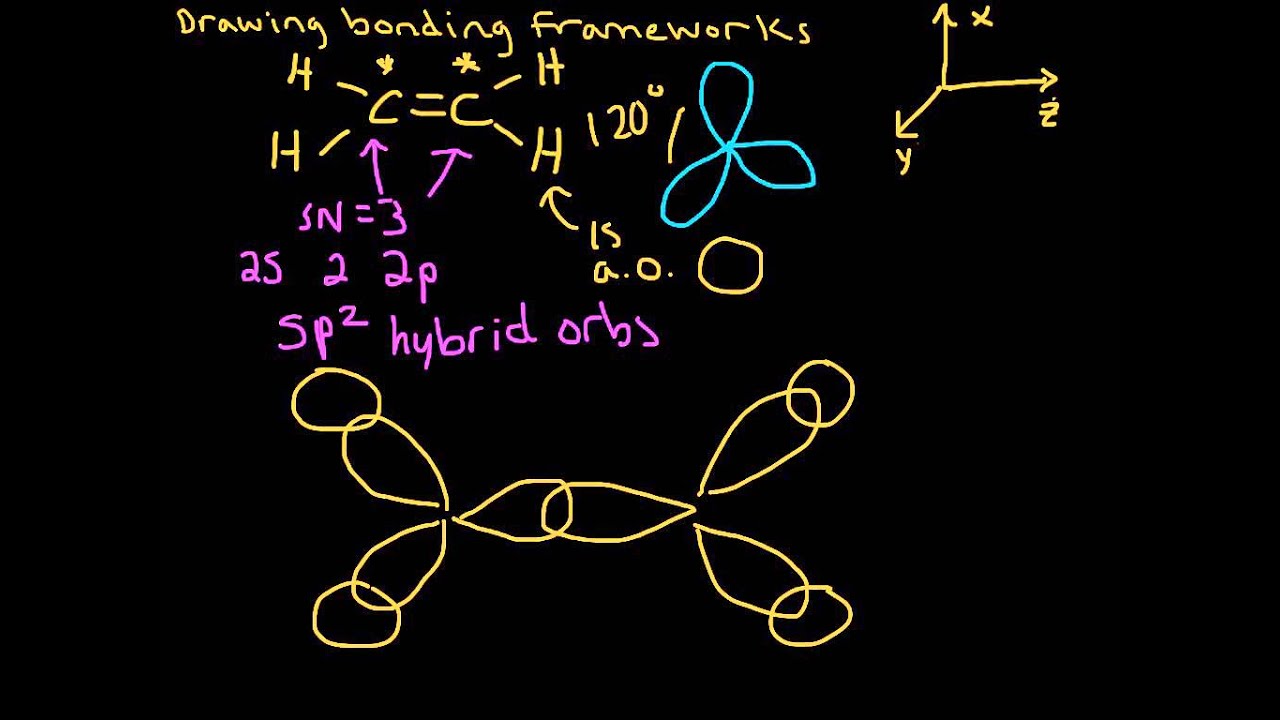
π- Molecular Orbitals (MO's) of Ethylene - Yale University 14 molecular orbitals read in model 2. 0 molecular orbitals read. Mulliken charges found for Model 2. Molecular dipole for model 2 = {0, 0, 0} Time for openFile (./mo/ethylene.log): 101 ms. reading 12 atoms. ModelSet: haveSymmetry:false haveUnitcells:false haveFractionalCoord:false. 2 models in this collection. Q. Draw molecular orbital diagram for ethane, ethene ... Sigma pi bond formation Orbital overlap concept ncert Molecular orbital energy level diagram for the two ... Molecular orbital energy level diagram for the two-ethylene system, before the molecules have come into contact, plotted with respect to the level degeneracy. π Molecular Orbitals of Ethene - chem.ucalgary.ca In chapter 1 we saw that the molecular orbitals of H2are created by the combination of 1s orbitals. The in-phase combination gave the bonding orbital. The out-of-phase combination the anti-bonding orbital. For ethene, the σ framework is created by the interaction of the sp2hybrid orbitals of the C atoms and H1s orbitals.
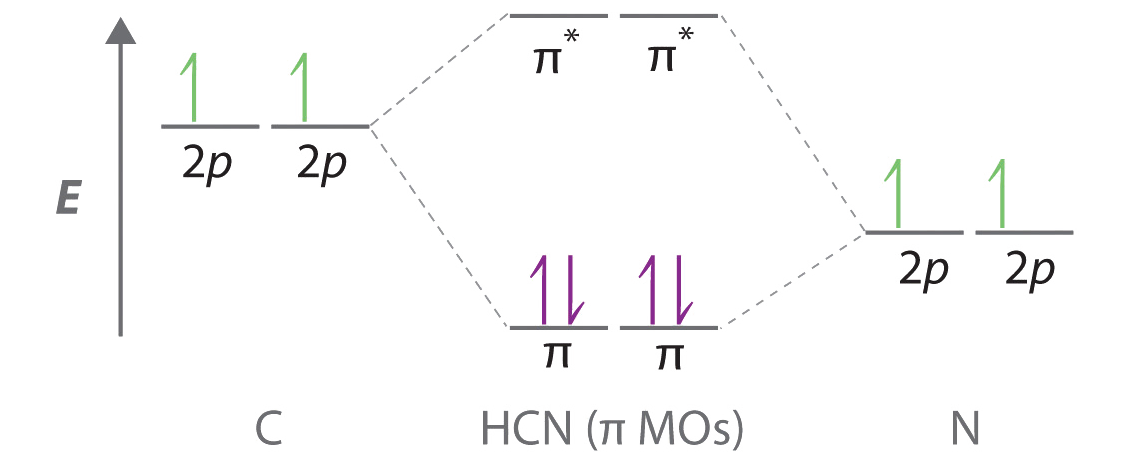
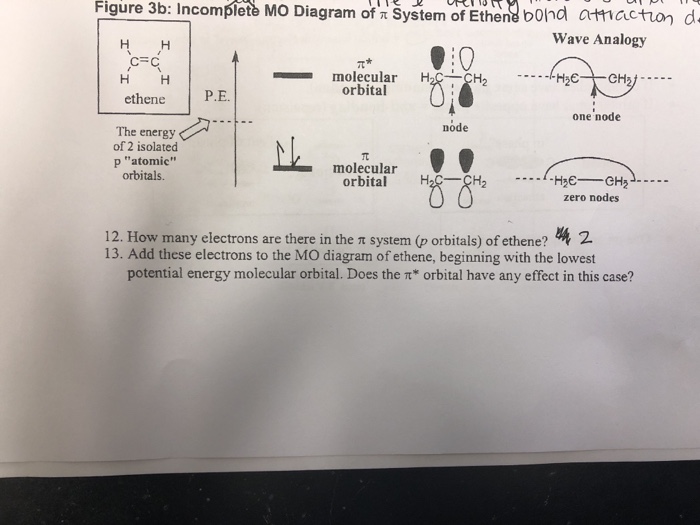
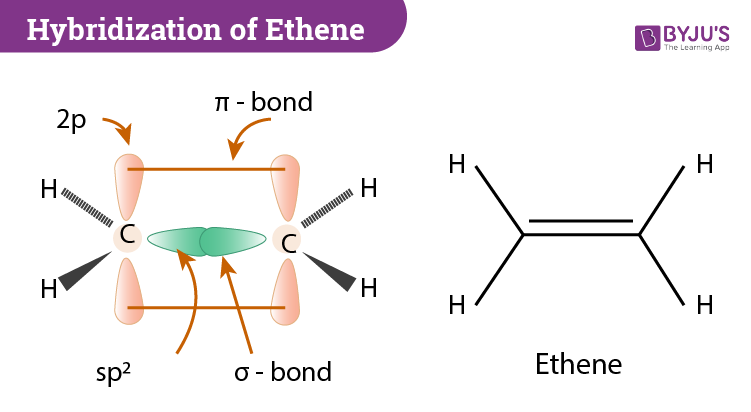
16.2a Introduction to Pi Molecular Orbitals Ethylene - YouTube Chad introduces Pi Molecular Orbitals using Ethylene, drawing Bonding and Antibonding Molecular Orbitals and identifying the HOMO and LUMO.I've created an or... Ethene Molecular Orbital Diagram - Wiring Diagrams A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons. PDF Pi Molecular Orbitals of Ethene The two electrons from the atomic p orbitals are now paired in the stabilised πι bonding orbital. This is the highest occupied molecular orbital or HOMOin ethene (or any simple alkene). In contrast, the π* anti-bonding orbital contains no electrons. It is the lowest unoccupied orbital or LUMO in ethene (or any simple alkene). Ethene-Orbital structure of ethene-reactions of ethene ... COMPOSITION OF ETHENE: Ethene molecule consists of two carbon atoms and four H-atoms i.e. CH2=CH2 NATURE OF HYBRIDIZATION: In ethene molecule each C-atom is Sp2-hybridized. Due to Sp2-hybridization each C-atom generates three Sp2-hybrid orbitals. In this way there exist six Sp2-hybrid orbital.
Molecular Orbital Diagram Of Ethene Ethene: The simplest alkene is ethene. Its chemistry is dominated by two "frontier orbitals", that is the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO). For the ethene orbital energy diagram these are shown as p CC for the HOMO, and p * CC for the LUMO.
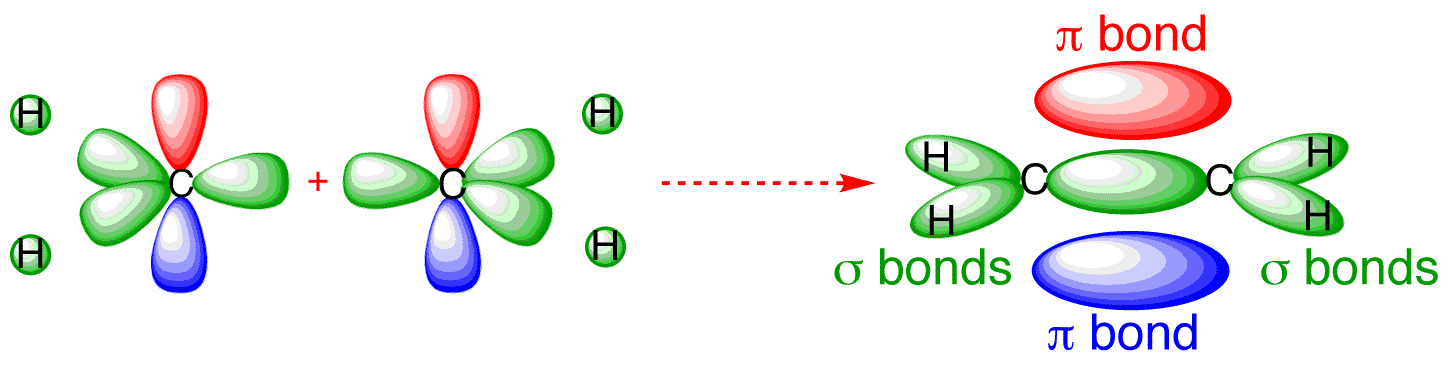
Hybridization of C2H4 (Ethene): Hybridization of Carbon in ... In ethene molecule, the carbon atoms are sp 2 hybridized. One unpaired electron in the p orbital remains unchanged. In ethylene, each carbon combines with three other atoms rather than four. There is a formation of a sigma bond and a pi bond between two carbon atoms. C2H4 Molecular Geometry And Bond Angles
42 ethylene molecular orbital diagram - Wiring Diagrams Manual Molecular Orbital Diagram Of Ethene Ethene: The simplest alkene is ethene. Its chemistry is dominated by two "frontier orbitals", that is the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO). For the ethene orbital energy diagram these are shown as p CC for the HOMO, and p * CC for the LUMO.
Interactions between Ethylene Molecular Orbitals and Metal ... Ethylene is capable of acting as a ligand as the C=C π bond can donate electron density to an empty metal d orbital, forming a σ bond. A filled metal d orbital is capable of donating electron density into the C=C π antibonding orbital. View Ethylene Molecular Orbitals here. Explore Metal-Ligand bonding with other molecules
Ethene Molecular Orbital Diagram - schematron.org Ethene: The simplest alkene is ethene. Its chemistry is dominated by two "frontier orbitals", that is the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO). For the ethene orbital energy diagram these are shown as p CC for the HOMO, and p * CC for the LUMO.
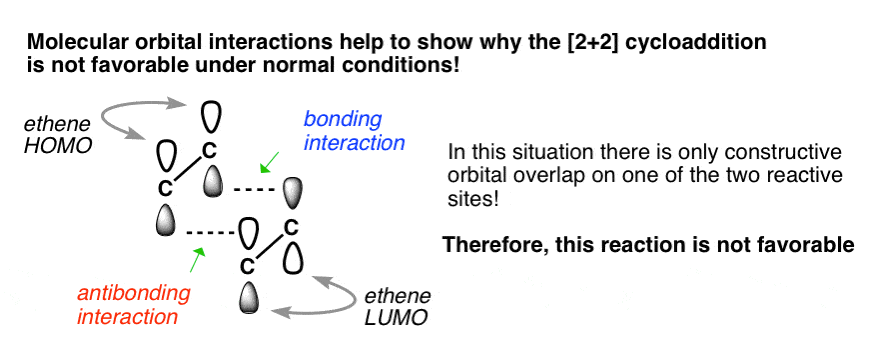
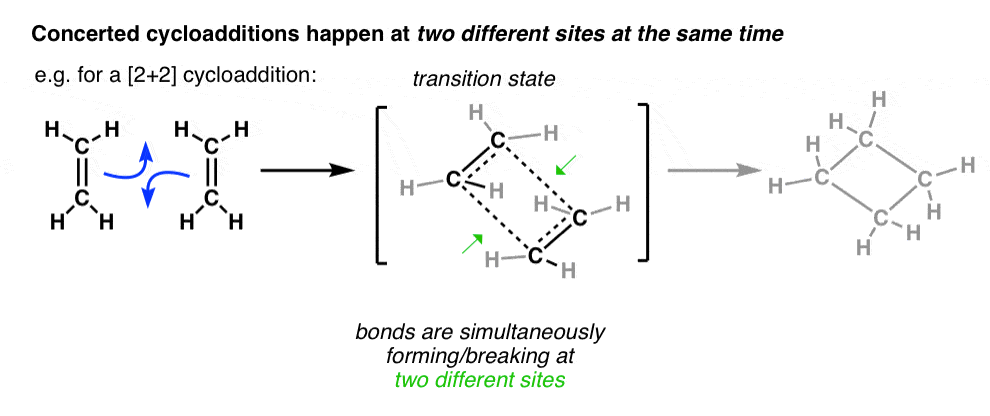
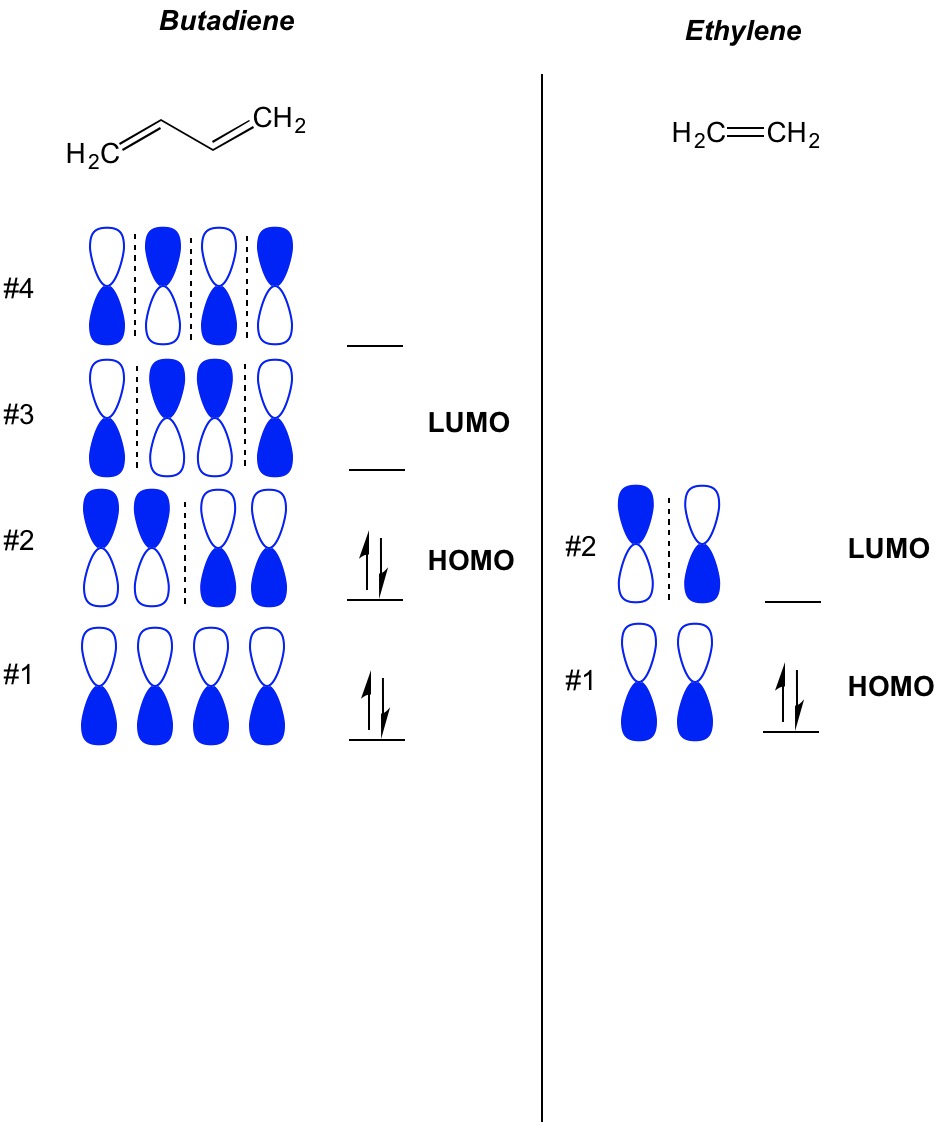
π Molecular Orbitals of Ethene Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" – interestingly, this reaction is rare and very slow !24 pages
Molecular Orbital Diagram Of Ethene A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons.
Molecular orbitals - Double bonds, Ethene This sideways overlap creates a molecular orbital known as a π-bond. A π-bond exists above and below the plane of the σ-bond, with a total of two electrons shared. This does not mean that one electron is above the plane and one is below the plane at any given time. The two electrons can exist anywhere in the π-bond at any given time.
Introduction to Molecular Orbital Theory Its chemistry is dominated by two "frontier orbitals", that is the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO). For the ethene orbital energy diagram these are shown as p CC for the HOMO, and p * CC for the LUMO.
Ethyne Molecular Orbital Diagram - Wiring Diagrams Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid . The carbon atoms in ethyne use 2sp hybrid orbitals to make their sigma bonds. After. Sigma (σ) bonding molecular orbital - Shared electron density is directly .. If we put all of the molecular orbitals of ethyne together, in a single energy diagram.
PDF The Huckel Approximation - Columbia University determine the shapes of the π molecular orbitals in ethylene. 6 Open the Hückel program. At the top of the window is a menu with the following options - File, Edit, View, Calculation, Help Below the menu is a tool bar. Below the tool bar is a page with a grid. Tool Bar
13.2. Molecular orbitals for ethene | Organic Chemistry II Molecular orbitals for ethene (ethylene) In the bonding pi orbital, the two shaded lobes of the p orbitals interact constructively with each other, as do the two unshaded lobes (remember, the arbitrary shading choice represents mathematical (+) and (-) signs for the mathematical wavefunction describing the orbital).
Molecular orbitals - Triple bonds, Ethyne Molecular orbitals - Double bonds, Ethene. Electronic configuration of atoms (Part 1) Next Top. ABPI. The Association of the British Pharmaceutical. Industry is a company limited by guarantee registered. in England and Wales (registered number 09826787) 7th Floor Southside, ...
Solved a) Considering the molecular orbital diagram for ... a) Considering the molecular orbital diagram for ethylene above, draw the molecules (with some kind of three-dimensional representation) of the initial interaction of ethylene with a very strong acid (represent this as H-A). b) Finish the molecular orbital diagram of formaldehyde (H 2 CO) by making the molecular orbitals in the middle. Don't ...











0 Response to "37 ethene molecular orbital diagram"
Post a Comment