39 orbital filling diagram for nitrogen
Filling of Electrons in Orbitals: Definition, Properties ... Filling of Electrons in Orbitals: Filing of Electrons in Orbitals: The representation of electrons in various shells, subshells, and orbitals is known as electronic configuration. The arrangement of electrons is of great importance in chemistry. In chemistry, electron configurations can be utilised to justify the chemical properties of elements. Orbital Filling Diagram For Nitrogen - schematron.org If that single electron were a spin-up (ms = +1/2), the orbital diagram for The figure below illustrating orbital diagrams for nitrogen is similar to the. Use orbital filling diagrams to describe the locations of electrons in an atom. Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p .
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
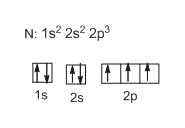
Orbital filling diagram for nitrogen
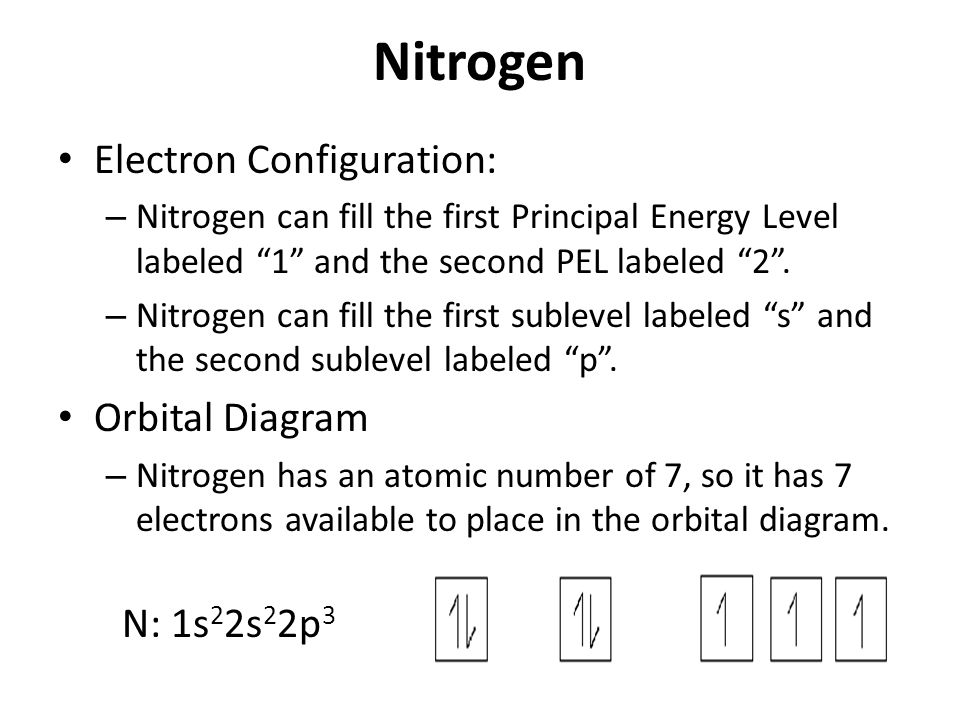
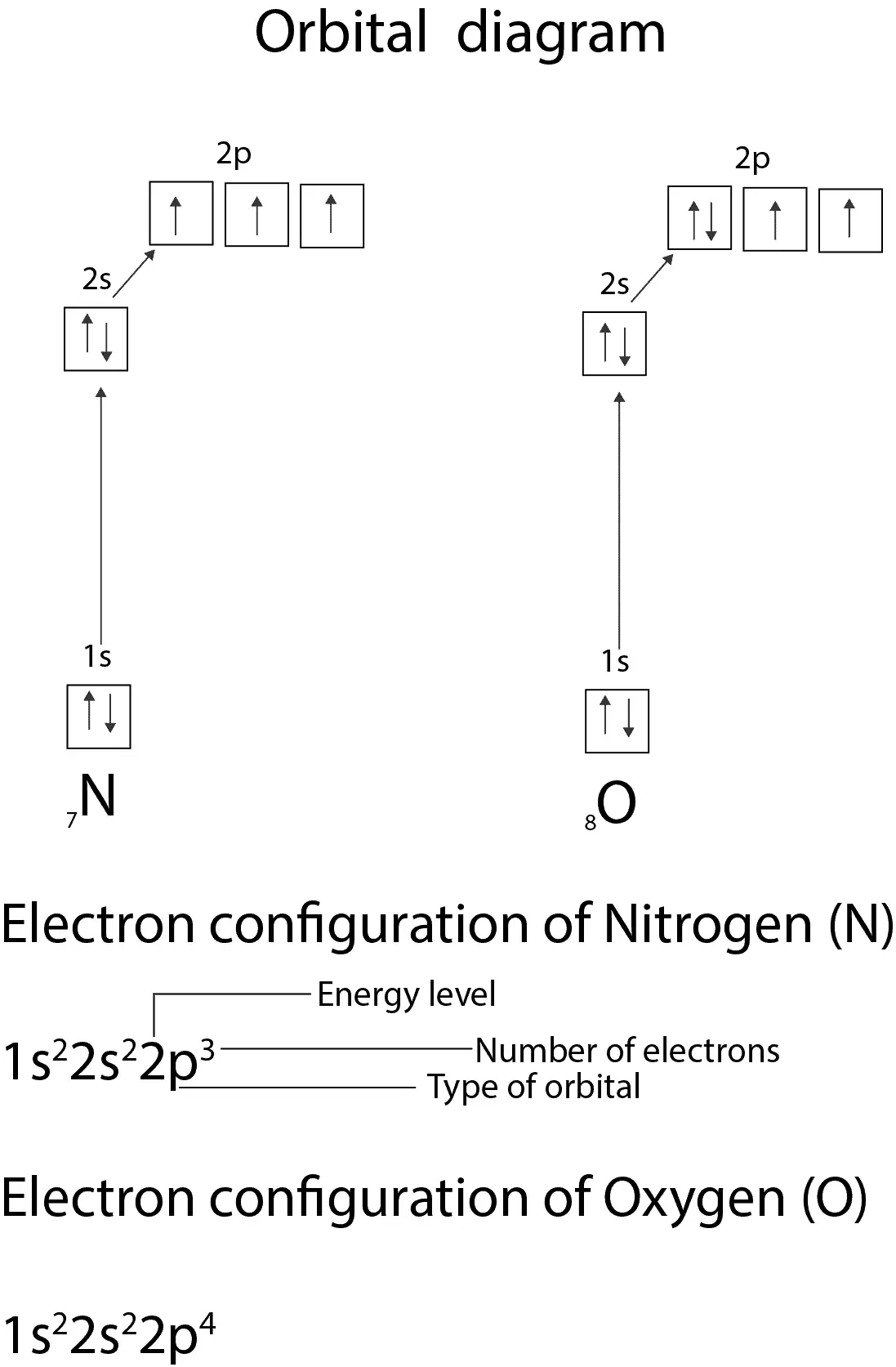
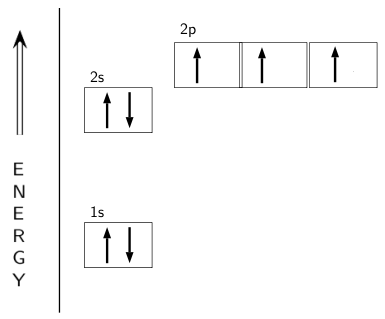
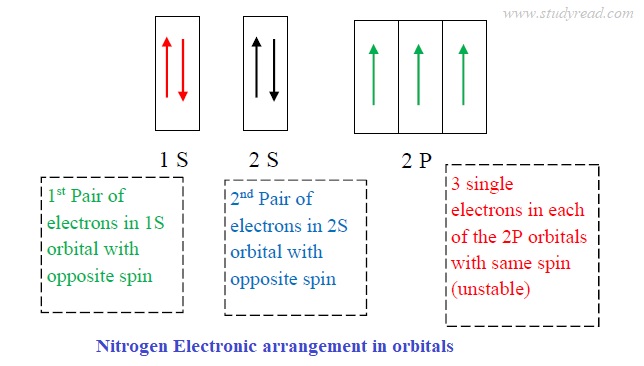
PDF Orbital Filling Diagrams - Oak Park USD Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Uparrow goes first then, downarrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Nitrogen Orbital diagram, Electron configuration, and ... The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and the rest three electrons in the 2p orbital. The orbital diagram for a ground-state electron configuration of a nitrogen atom is as follows- Orbital Filling Diagram For Nitrogen In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. Use orbital filling diagrams to describe the locations of electrons in an atom.
Orbital filling diagram for nitrogen. Fill In The Atomic Orbital Diagram For Nitrogen - hyundai Fill In The Atomic Orbital Diagram For Nitrogen Fill In The Atomic Orbital Diagram For Nitrogen - All over the 1980s, Hyundai saw rapid development, making sizeable inroads into worldwide marketplaces. Nevertheless, until finally 1986, the company reached amongst its principal targets: breaking in the American market. What is the orbital diagram of nitrogen? Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Nitrogen Electron Configuration (N) with Orbital Diagram Jan 21, 2021 — When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next ... Orbital Diagrams Flashcards - Quizlet An orbital can hold a maximum of 2 electrons. to occupy the same orbital the electrons must spin opposite directions Hund's Rule within a sub level, place one electron per orbital before pairing them "empty bus seat rule" Orbital filling diagram for carbon Orbital filling diagram for nitrogen Aufbau Principle
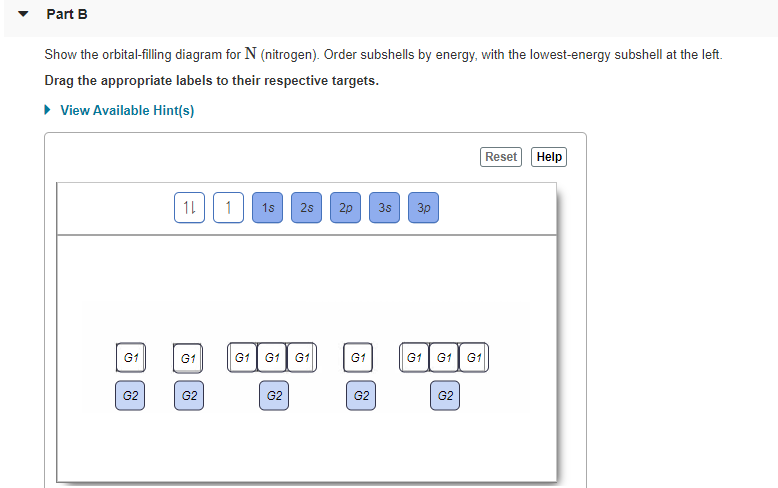
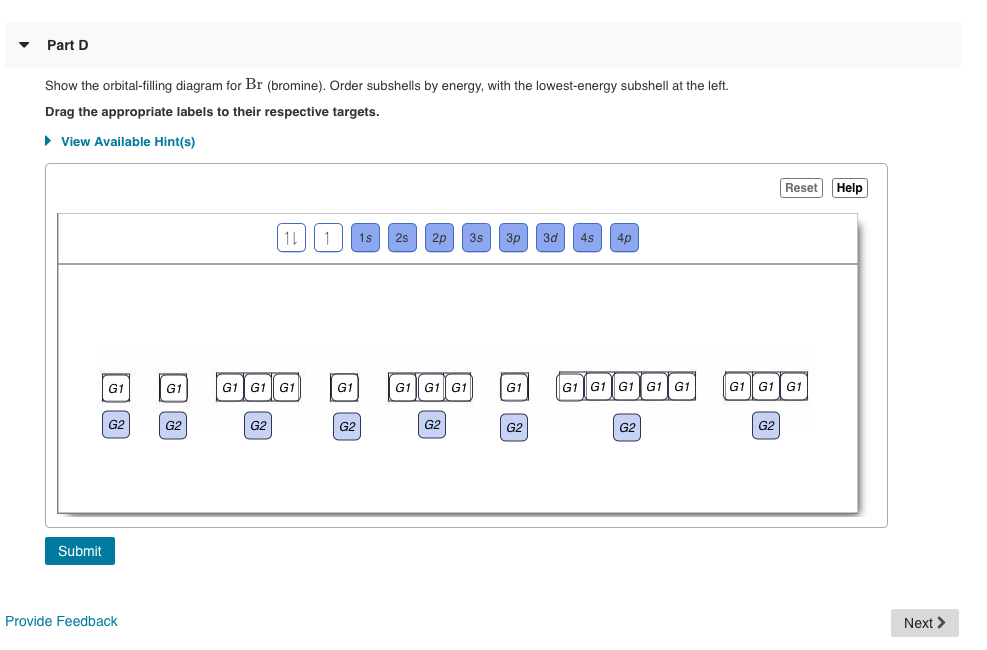
Solved Show the orbital-filling diagram for N (nitrogen ... Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. Nitrogen(N) electron configuration and orbital diagram Orbital Diagram for Nitrogen Electron configuration of nitrogen in the excited state Atoms can jump from one orbital to another in an excited state. This is called quantum jump. The ground-state electron configuration of nitrogen is 1s 2 2s 2 2p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Orbital Filling Diagram For Boron - schematron.org Show the orbital-filling diagram for N (nitrogen)% (8). The outer-most electrons are the only ones included in the orbital filling diagram and the electron dot diagram because the outer-most electrons are the only ones that need to be used in chemical reactions and bonding, so the other electrons are insignificant in these diagrams. 5.17 Hund's Rule and Orbital Filling Diagrams Flashcards ... The number of unpaired electrons in an atom can be determined from the orbital filling diagrams and correctly applying Hund's rule. Boron has one unpaired electron, while carbon has two, and nitrogen has three. How many unpaired electrons does fluorine have? 1. For the principal quantum (shell) number of n equal 2, we can have ____ sub-levels. ...
Periodic Trends Flashcards - Quizlet Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. 1s²2s²2p³ Item 2: Part C Show the orbital-filling diagram for S (sulfur). Answered: Show the orbital-filling diagram for N… | bartleby Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint (s) Reset Help 1L 1 1s 2s 2p 3s Зр G1 G1 G1 G1 G1 G1 G1 G1 G1 G2 G2 G2 G2 G2 Part C Show the orbital-filling diagram for S (sulfur). Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... There are 5 valence electrons in Nitrogen Electron Configuration and it lies at the top of group 15 in the periodic table. Apart from that one more thing is unique about the element, i.e, nitrogen can have either one of 3 or 5 valence electrons. Solved Show the orbital-filling diagram for N (nitrogen ... Chemistry questions and answers. Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left Drag the appropriate labels to their respective targets. View Available Hint (s) Reset Help 11 || 1 18 2s 2p 3s ap Group 1 GT G1 G1 G1 GI GT G161 G2 G2 G2 G2 G2.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
Electron Configuration Orbital Diagram Nitrogen - YouTube To see this video, other videos, chemistry education text, and practice problems visit my website. Website is 100% FREE to use.
Hund's Rule and Orbital Filling Diagrams - Lumen ... The Figure below shows how a set of three p orbitals is filled with one, two, three, and four electrons. Figure 1. The 2p sublevel, for the elements boron (Z = 5), carbon (Z = 6), nitrogen (Z = 7), and oxygen (Z = 8).
Answered: Show the orbital-filling diagram for N… | bartleby Transcribed Image Text: IReview I Constants I Periodic Table Nine orbitals (one s, three p, and five d) can hold a maximum of 18 electrons. Part B Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left.
How to Draw Orbital Diagrams for any atom | Orbital Notation We know the nitrogen has a total of 7 electrons that need to be placed into orbitals, now for drawing its orbital diagram, we need to show its electrons in form of an arrow in different boxes using Aufbau, Hund's, and Pauli's exclusion rule. Nitrogen has a total of 7 electrons and its electron configuration is 1s 2 2s 2 2p 3.
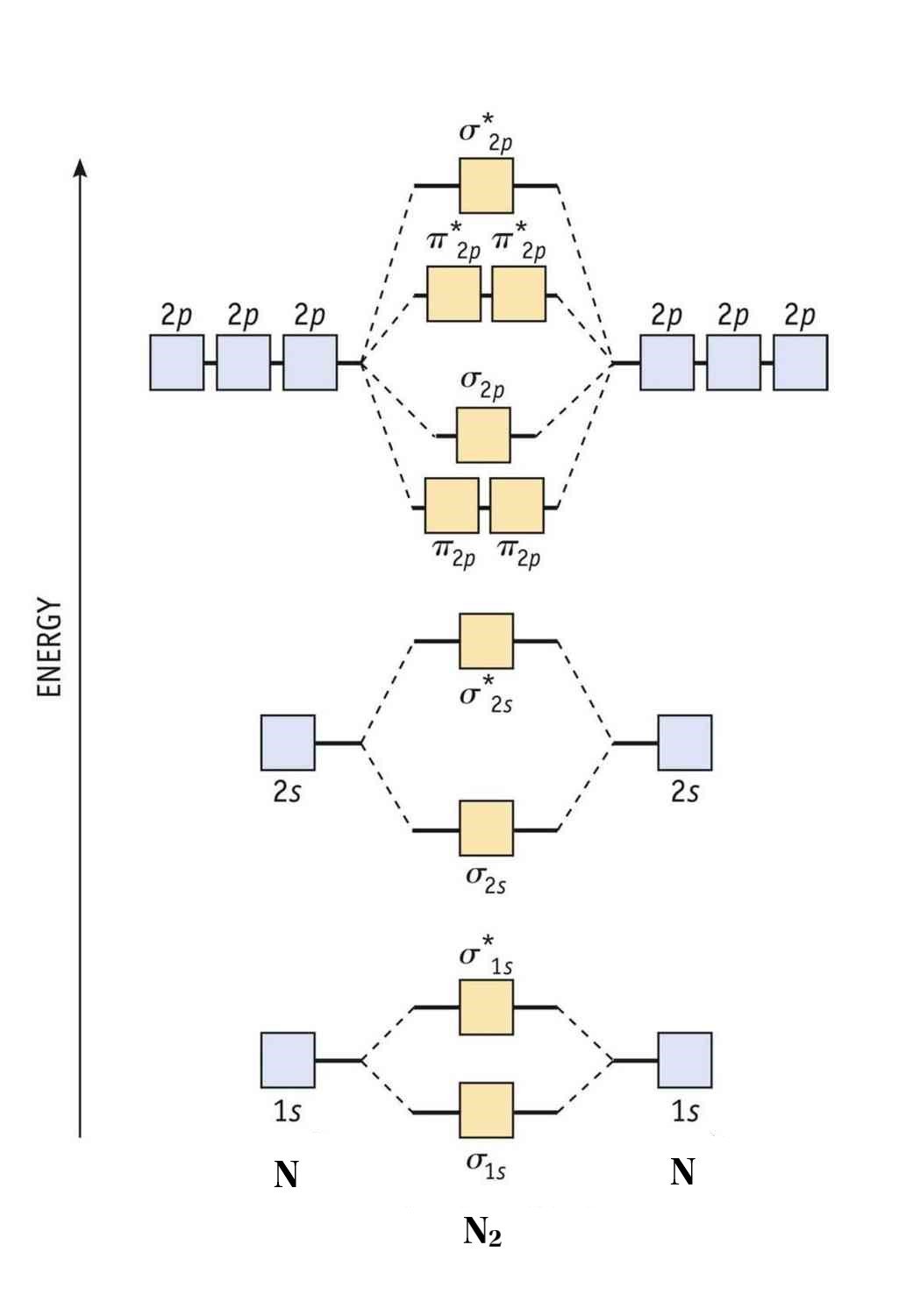
8 - Drawing Molecular Orbital Diagrams — Flux Science To fill the diagram, first, we fill each side of the diagram with the electrons according to nitrogen's electron configuration - [He]2s 2 2p 3. Next, we fill the middle section with the molecular orbital's electron configuration using Hund's Rules, just as we do with atomic orbitals. We fill each shell with two electrons before moving to ...
Molecular Orbital (MO) Diagram of N2 - YouTube Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
Filling of Orbitals in Atom - Self Study Point Filling of Orbitals in Atom. The filling of electrons into the orbitals of different atoms takes place according to the aufbau principle which is based on the Pauli's exclusion principle, the Hund's rule of maximum multiplicity and the relative energies of the orbitals.. Aufbau Principle. The word 'aufbau' in German means 'building up'.The building up of orbitals means the filling ...
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital.
5.17: Hund's Rule and Orbital Filling Diagrams - Chemistry ... Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.
Molecular orbital energy level diagrams -Hydrogen ... Molecular orbital energy level diagrams -Hydrogen, Hypothetical, Nitrogen, Oxygen. The filling of molecular orbitals is governed by the following principles. (i)Aufbau principle (ii)Pauli's exclusion principle and (iii)Hund's rule of maximum multiplicity. Now, let us consider some examples of homo nuclear diatomic molecules.
Orbital Filling Diagram For Nitrogen In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. Use orbital filling diagrams to describe the locations of electrons in an atom.
Nitrogen Orbital diagram, Electron configuration, and ... The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and the rest three electrons in the 2p orbital. The orbital diagram for a ground-state electron configuration of a nitrogen atom is as follows-
PDF Orbital Filling Diagrams - Oak Park USD Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Uparrow goes first then, downarrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom

























0 Response to "39 orbital filling diagram for nitrogen"
Post a Comment