35 solidus line phase diagram
A phase diagram (or equilibrium diagram) is a diagram with T and composition as axes, showing the equilibrium constitution. The phase diagram of an alloy made of components A and B, for all combinations of T and X B, defines the A-B system. Binary systems have two components, ternary systems three, and so on. Commercial alloys may Phase Diagrams. 1 component systems that can have multiple phases, though the equilibrium solid phase is a crystal; depend on temperature and pressure alone. multi-component systems with multiple phases. specifically, it can have multiple equilibrium solid phases possible at a given temperature. Depends on temperature, pressure, and composition.
In this case the diagram is read *up-across-down* (following the numbers. 1. Begin with an original composition of 60% anorthite. 2. Heat the rock until it hits the solidus line. Here the first drop of liquid appears as the rock starts to melt. 3. To find the composition of the first melt draw a ling straight across horizontally to the liquidus line.
Solidus line phase diagram
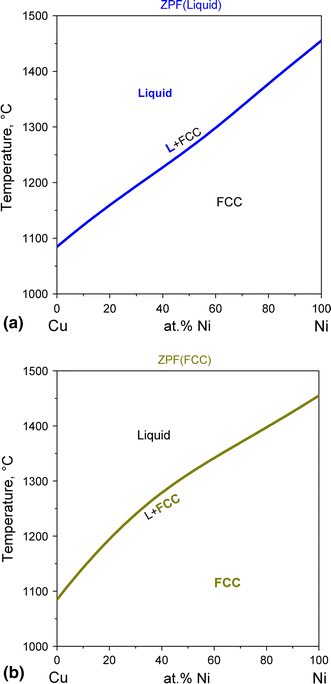
3. The degree of freedom at triple point in unary diagram for water _____. (a) 0 (b) 1 (c) 2 (d) 3 4. Above the following line, liquid phase exist for all compositions in a phase diagram. (a) Tie-line (b) Solvus (c) Solidus (d) Liquidus 5. Following is wrong about a phase diagram. (a) It gives information on transformation rates. The region between the liquidus line and the solidus line is also referred to as a "two phase region"! How to read a phase diagram Using a copper-nickel alloy consisting of 55 % nickel (CuNi55 alloy) as an example, the interpretation of the phase diagram at different temperatures is explained in more detail below. in 2-phase region: 1. Draw the tie line. 2. Note where the tie line intersects the liquidus and solidus lines (i.e. where the tie line crosses the phase boundaries). 3. Read off the composition at the boundaries: Liquid is composed of CL amount of Ni (31.5 wt% Ni). Solid is composed of Cαααα amount of Ni (42.5 wt% Ni).
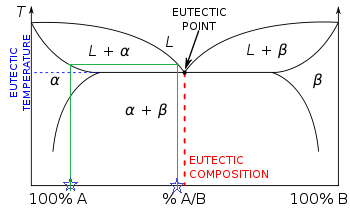
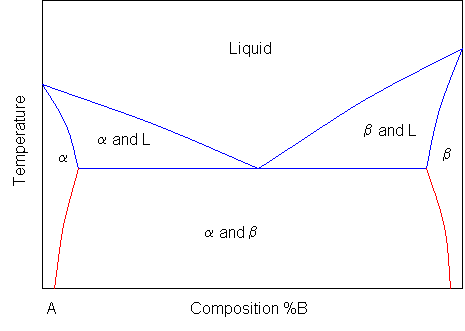
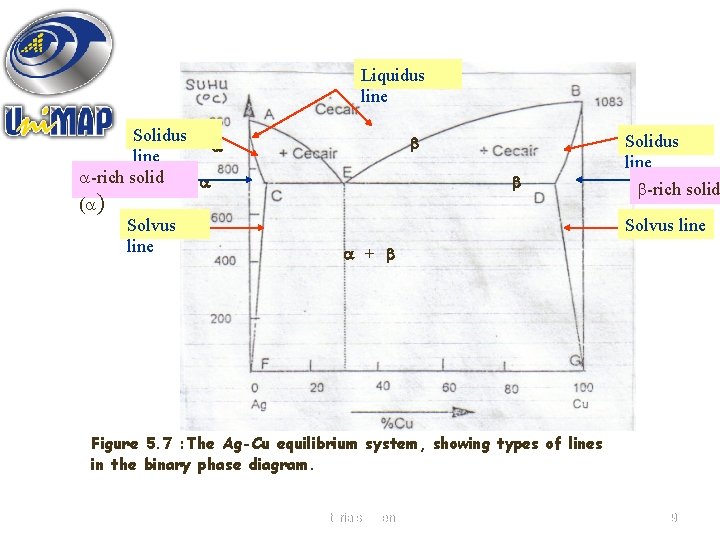
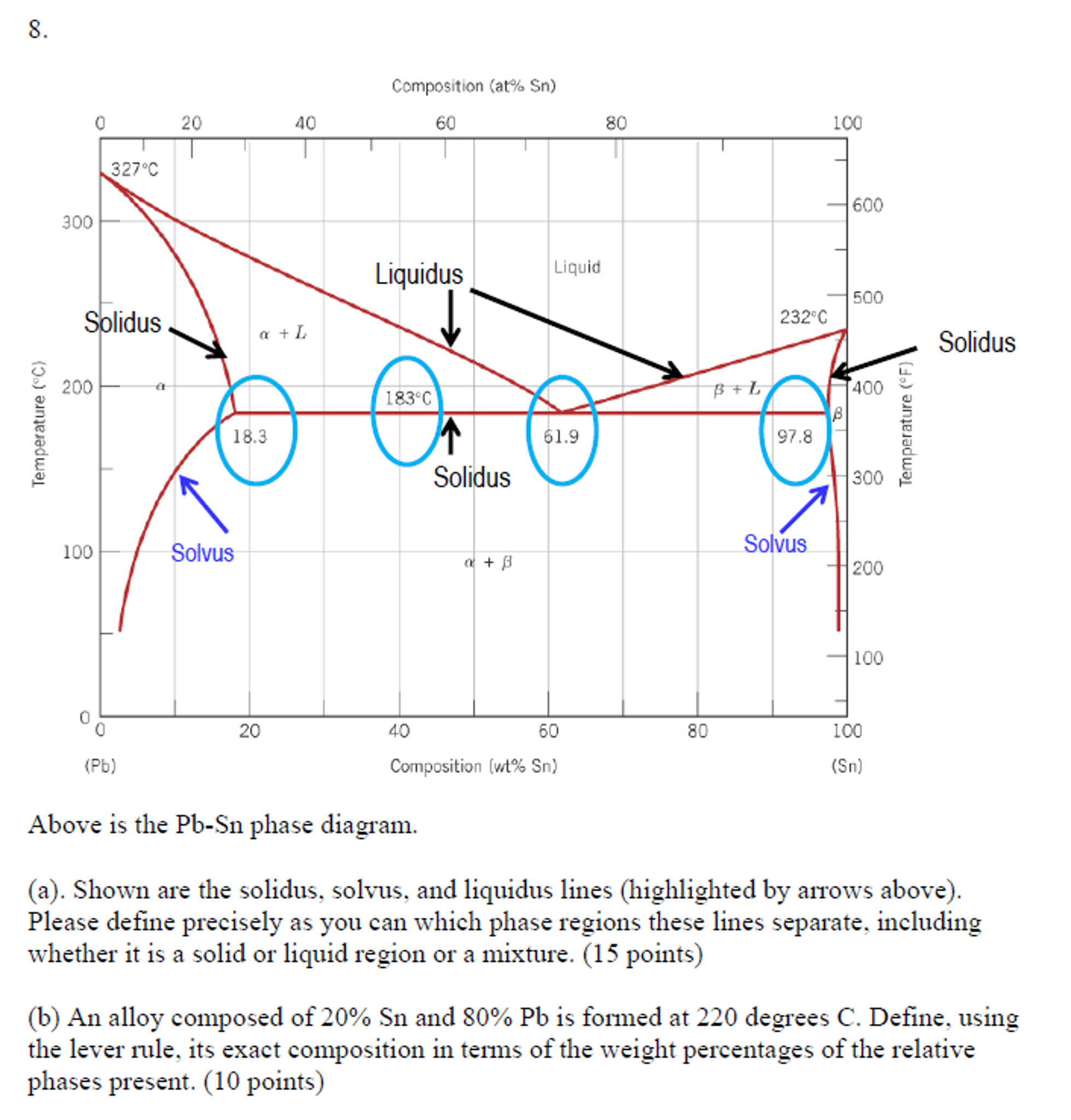
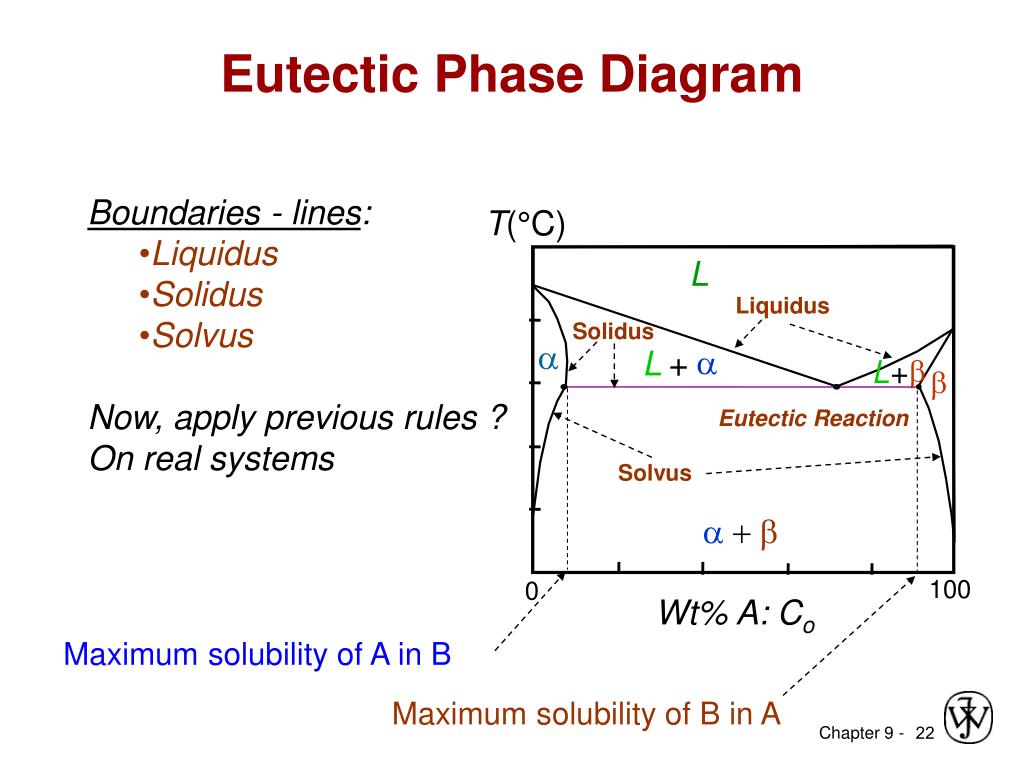
Solidus line phase diagram. The solvus is represented by a line on a phase diagram that separates a solid phase from a solid1 + solid2 phase, where solid1 and solid2 are different microstructures. The eutectic is represented by the horizontal line in a eutectic binary phase diagram, connecting the intersections of the solidus and solvus lines from both sides. The eutectic temperature also is where the liquidus lines for both components meet. This phase diagram consists of two points, two lines and three areas. The two points of the two pure metals A & B. The upper line, obtained by connecting the points showing the beginning of solidification is called liquidius line, and the lower line, determined by connecting the points showing the end of solidification is called the solidus line. Liquidus - The line separating the field of all liquid from that of liquid plus crystals. Solidus - The line separating the field of all solid from that of liquid plus crystals. Eutectic point - the point on a phase diagram where the maximum number of allowable phases are in equilibrium. When this point is reached, the temperature must remain ... Solidus line. The temperature occurring as a function of the composition of an alloy at which the cooling melt of this alloy completely solidifies is known as its solidus line (analogous to the liquidus line) . Between these two lines, a mixture of the metals is present in liquid and solid phases. When a eutectic alloy reaches the solidus line ...
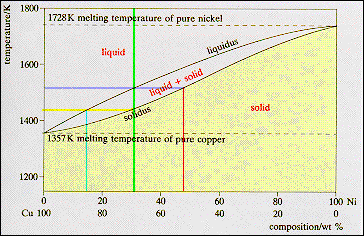
Solidus: Solidus is a line on the phase diagram below which the substance is completely solid. The corresponding temperature is known as the solidus temperature. Solvus: It is defined as a curve on a phase diagram that shows the limits of solubility of one solid phase in another. It is a function of temperature. 3. Different 3 -phase fields do NOT meet, except possibly at a point. 4. 3 -phase fields and 2 phase fields are separated from each other by straight lines. (See 1 and 2 above. ) 5. 2- and I -phase fields are usually separated from each other by curved lines . 6. Concerning the extrapolation of 3 -phase A and 2 -phase lines : 3- and I -phase ... The equilibrium "phase diagram" opposite shows the dependence of the alloys melting point on its composition, and the existence of the single solid phase below the solidus line indicates the complete solid state miscibility of the two elements. The phase diagram shows that the melting point of each of the pure elements is a unique value: 1357 K ... Liquidus and solidus projections of the Ti-TiNi-TiRu phase diagram: ∘ a two-phased alloy • three-phased alloy EMPA data of compositions of conjugated phases. p 1 peritectic point. p 2 peritectic point. e 1 eutectic point (∼24 at. % Ni at 942°C) e minimum point in the solidus and liquidus curves. ∼ 16 at.% Ru at 1550°C
phase diagram A(1100, 60): 1 phase: B(1250, 35): 2 phases: L + Determination of phase(s) present Melting points: Cu = 1085°C, Ni = 1453 °C Solidus - Temperature where alloy is completely solid. Above this line, liquefaction begins. Liquidus - Temperature where alloy is completely liquid. Below this line, solidification begins. In Cigar shape diagram there are three different regions: 1. Liquid (single phase) 2. Liquid + solid (double phase) 3. Solid solution (single phase) •Liquidus- the boundary line between the liquid region and the double phase region. •Solidus- the boundary line between the solid solution region and the double phase region. 2. It is slowly heated until it reaches the solidus line. At the solidus line the system shifts laterally to the 30/70 eutectic point. 3. At the eutectic both components P and Q begin melting at the same time in the ratio of 30% P and 70% Q. Melting at the eutectic is always at the ratio of the eutectic, regardless of the starting composition. 1. Locate composition and temperature in diagram . 2. In two phase region draw the tie line or isotherm . 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line . The lever rule is a mechanical analogy to the mass balance calculation. The tie line
Solidus Line: On a phase diagram, the locus of points at which solidification is complete upon equilibrium cooling, or at which melting begins upon equilibrium heating. Solubility: The amount of substance that will dissolve in a given amount of another substance.
In Cigar shape diagram there are three different regions: 1. Liquid (single phase) 2. Liquid + solid (double phase) 3. Solid solution (single phase) •Liquidus- the boundary line between the liquid region and the double phase region. •Solidus- the boundary line between the solid solution region and the double phase region.

Pharmaceutics Free Full Text Drug Solubility Enhancement Through The Preparation Of Multicomponent Organic Materials Eutectics Of Lovastatin With Carboxylic Acids Html
The solid solubility of Bi in Cu single crystals has been experimentally determined. It is shown that the solidus line is a retrograde curve without a monotectic transition. The solid and liquid phases are successfully described with simple thermodynamic models. The experimentally measured maximum solubility of 0.0207 at. % Bi at 975 °C correlates well with that from the model (0.0193 at ...
Solidus is a see also of liquidus. Liquidus is a see also of solidus. In context|chemistry|physics|lang=en terms the difference between liquidus and solidus is that liquidus is (chemistry|physics) a line, in a phase diagram, above which a given substance is a stable liquid and below which solid and liquid are in equilibrium while solidus is (chemistry|physics) a line, in a phase diagram, below ...
Solidus line is shifted to the right (higher Ni contents), solidification is complete at lower T, the outer part of the grains are richer in the low-melting component (Cu). • Upon heating grain boundaries will melt first. This can ... Copper - Silver phase diagram liquid α+ β Solidus
The phase diagram, therefore, consists of a liquidus line showing a minimum at the eutectic temperature, which is itself marked by a horizontal line. Since the solid phases formed consist simply of pure cadmium or pure bismuth, the solidus lines are coincident with the two vertical temperature axes. 1.14.
Shown is a partial binary phase diagram for the Copper-Nickel system with the alloy Cu-35 wt% Ni at the vertical line. At the point, a, the alloy is a liquid. Upon cooling it passes through the two-phase (a + L) zone and then solidifies as a single phase substitutional alloy. Between (b) where the composition line intersects the liquidus line ...
Answer: > liquidus is represented by a line on a phase diagram that separates a liquid phase from a solid + liquid phase region. A system must be heated above the liquidus temperature to become completely liquid. The liquid system begins to solidify when the temperature cools to the liquidus tem...
This is referred to as the solidus temperature, and the solidus line describes this temperature at varying compositions. The liquidous line describes the temperature at which the last little bit of solid melts at varying compositions. The eutectic line is a horizontal line drawn between two one-phase fields a the eutectic temperature.
Binary phase diagrams are important tools for designing desired alloys. In the Sn-rich region of the Sn-In alloy phase diagram, the solidus line appears as a dotted line in current literature plots to indicate uncertainty. The contour of the solidus has now been clarified as a result of the present investigation. Four alloys, Sn70In30, Sn75In25, Sn80In20, and Sn85In15 were melted at 300 °C ...
Two component TX phase diagrams for solid solutions have three principal divariant areas separated by univariant lines. Above the liquidus line the system is entirely molten and below the solidus line it is entirely solid. Note that the solid is a single mineral (one phase) but it is a mixture - it is impure.
The phase diagram shows, in pressure–temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas. The curves on the phase diagram show the points where the free energy (and other derived properties) becomes non-analytic: their derivatives with respect to the coordinates (temperature and pressure in this example) change discontinuously (abruptly).
system, the phase diagram usually has the general appearance of that shown in Fig. 3. The diagram consists of two single-phase fields separated by a two-phase field. The boundary between the liquid field and the two-phase field in Fig. 3 is called the liquidus; that between the two-phase field and solid field is the solidus.
John F. Smith, in Methods for Phase Diagram Determination, 2007. Publisher Summary. It was recognized that the terminal solubilities of impurities in a material differ between the liquid and solid states. In a phase diagram, this would be indicated by the separation between the liquidus and solidus lines. The fact that industry believes in the importance of phase diagrams is proven by the fact that industrial funding made the alloy phase diagram program possible.
In chemistry, materials science, and physics, the solidus is the locus of temperatures (a curve on a phase diagram) below which a given substance is completely solid (crystallized). The solidus temperature, T S or T sol, specifies the temperature below which a material is completely solid, and the minimum temperature at which a melt can co-exist with crystals in thermodynamic equilibrium.
Question & Answer. Q: Where does the solidification in a phase diagram start? A) Liquidus line. B) Solidus line. C) At equilibrium.
in 2-phase region: 1. Draw the tie line. 2. Note where the tie line intersects the liquidus and solidus lines (i.e. where the tie line crosses the phase boundaries). 3. Read off the composition at the boundaries: Liquid is composed of CL amount of Ni (31.5 wt% Ni). Solid is composed of Cαααα amount of Ni (42.5 wt% Ni).
The region between the liquidus line and the solidus line is also referred to as a "two phase region"! How to read a phase diagram Using a copper-nickel alloy consisting of 55 % nickel (CuNi55 alloy) as an example, the interpretation of the phase diagram at different temperatures is explained in more detail below.
3. The degree of freedom at triple point in unary diagram for water _____. (a) 0 (b) 1 (c) 2 (d) 3 4. Above the following line, liquid phase exist for all compositions in a phase diagram. (a) Tie-line (b) Solvus (c) Solidus (d) Liquidus 5. Following is wrong about a phase diagram. (a) It gives information on transformation rates.












0 Response to "35 solidus line phase diagram"
Post a Comment