36 lewis dot diagram for hcn
13 Jun 2019 — HCN has a linear geometry but unlike nonpolar CO2 is polar due to the intrinsic electronegativity on nitrogen and the stronger force by ... 15/11/2021 · CN Lewis Structure What is Lewis Structure? If we have a look into the above-mentioned diagram, we can see that it is a sketch of an oxygen atom. Here since an oxygen atom has an atomic number of 8, we have six electrons in the outermost shell. The outermost shell is known as the valence shell that determines the valency i.e the combining capacity of the atom with other atoms for molecule ...
10/11/2021 · Lewis structure is also known as electron dot structure or Lewis dot structure because the valence electrons are represented as dots in the Lewis structure of the molecule. It is the two-dimensional structure in which every atom in the molecule tends to complete its octet either by sharing or gaining or losing electrons. However, there are some exceptions as well. For example, Hydrogen, …
Lewis dot diagram for hcn
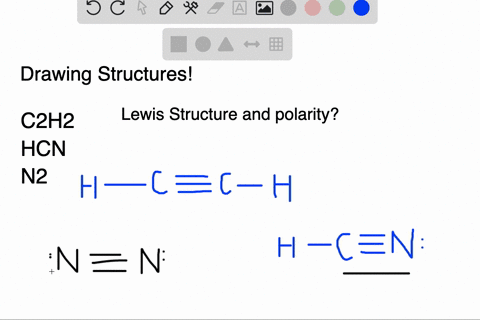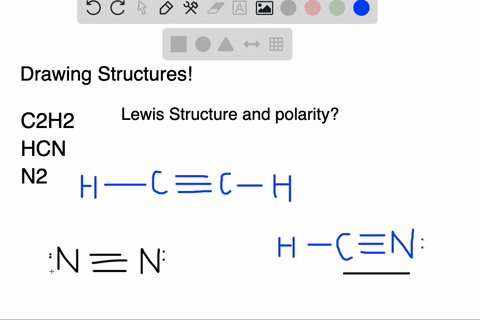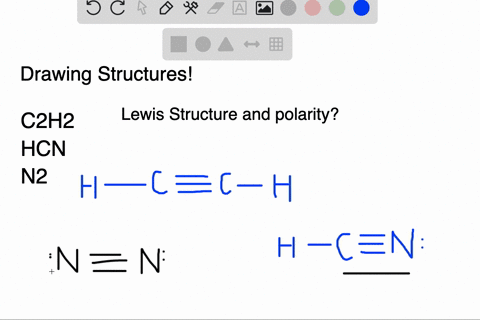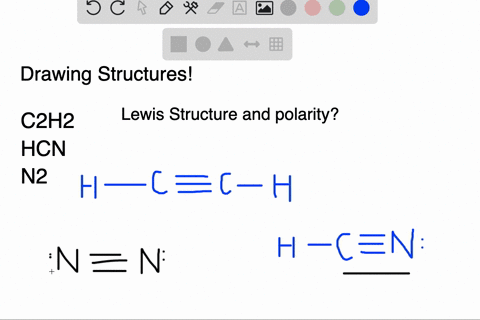
We have a total of ten valence electrons for the HCN Lewis structure. We'll put two between atoms to form chemical bonds, so we've used four, then we'll go ...1 Oct 2013 · Uploaded by Wayne Breslyn In this case, you can't really draw a symmetrical structure since there are three atoms. All possible linear structures are shown below. H-C-N H-N-C C-H-N. • (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding. • Use valence shell electron pair repulsion (VSEPR) model to ...
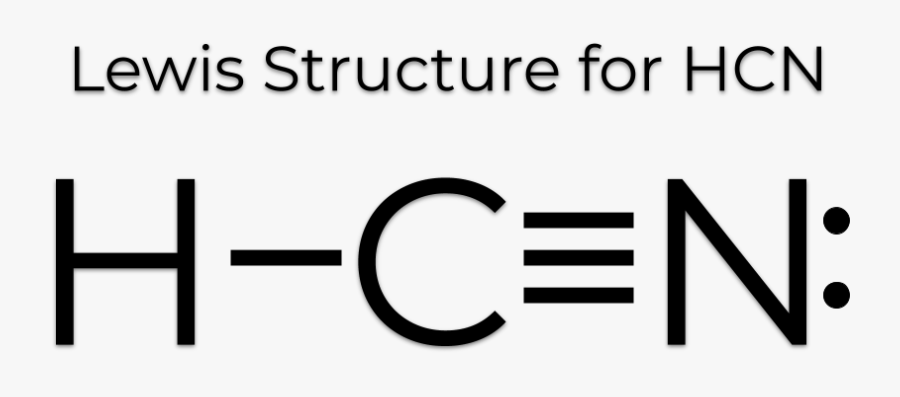
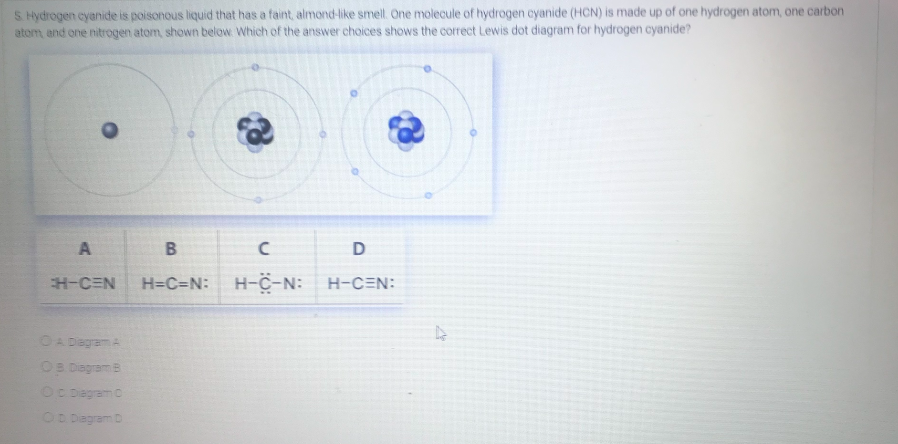
Lewis dot diagram for hcn. Once you get the total number of valence electrons, you can make a Lewis dot structure of HCN. This structure helps in understanding the ...23 Jul 2021 · Uploaded by Geometry of Molecules 1 answerThe Lewis structure of hydrogen cyanide is shown below. This is a linear compound due to the presence of a triple bond between carbon and nitrogen... Conclusion · HCN is a highly toxic substance that has a bitter almond-like smell. · There is one bond between H and C and three bonds between C and nitrogen. In case of $HCN$ valence electrons are 10 as Hydrogen have valency 1, Carbon 4 and Nitrogen 5. 2. Now we have to find the octet electron for each atom and add ...
• (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding. • Use valence shell electron pair repulsion (VSEPR) model to ... In this case, you can't really draw a symmetrical structure since there are three atoms. All possible linear structures are shown below. H-C-N H-N-C C-H-N. We have a total of ten valence electrons for the HCN Lewis structure. We'll put two between atoms to form chemical bonds, so we've used four, then we'll go ...1 Oct 2013 · Uploaded by Wayne Breslyn

Molecular Formula Lewis Dot Structure Number Of Electron Molecular Geometry Domains Hcn 1 4 5 H Homeworklib

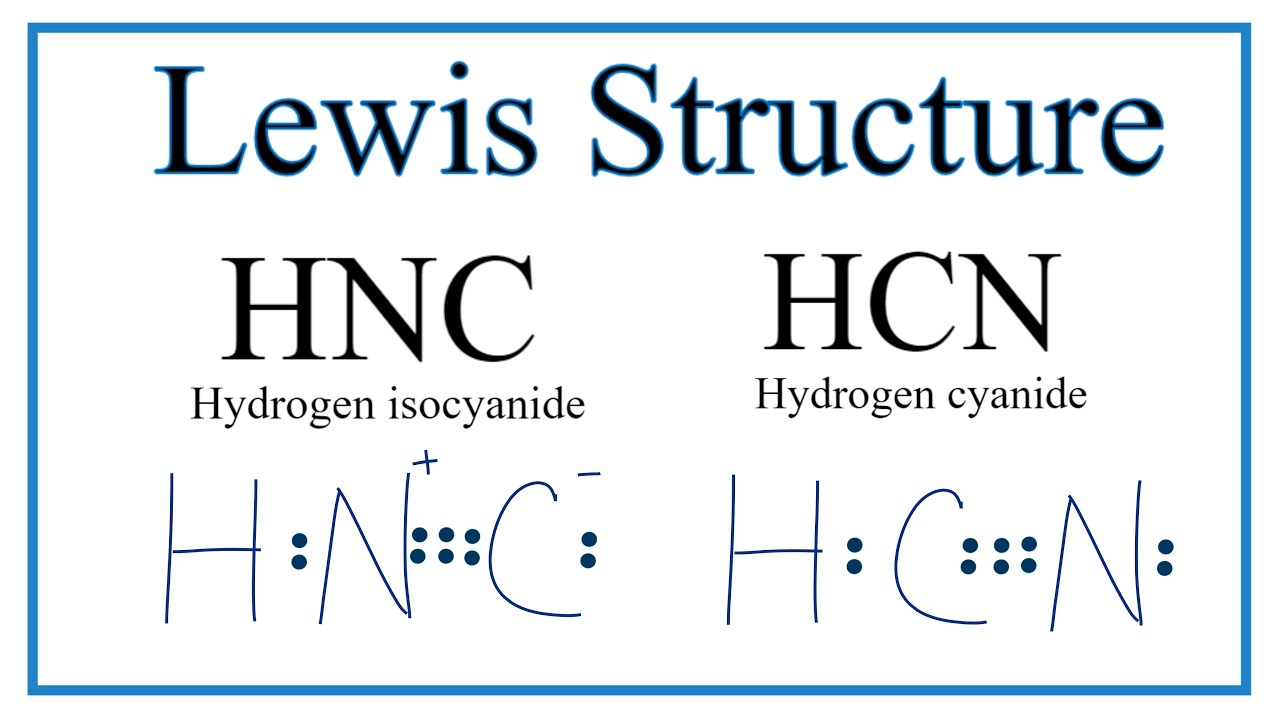
Solved Hydrogen Isocyanide Hnc Has The Same Elemental Composition As Hydrogen Cyanide Hcn But The H Atom In Hnc Is Bonded To The Nitrogen Atom Draw A Lewis Structure For Hnc And Assign
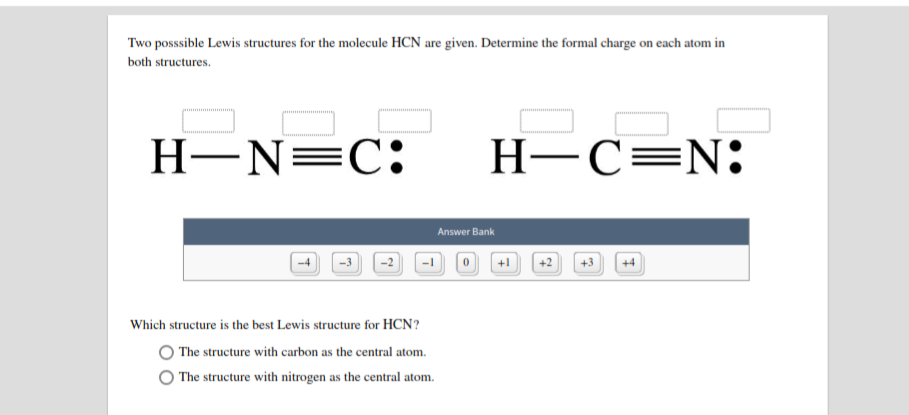
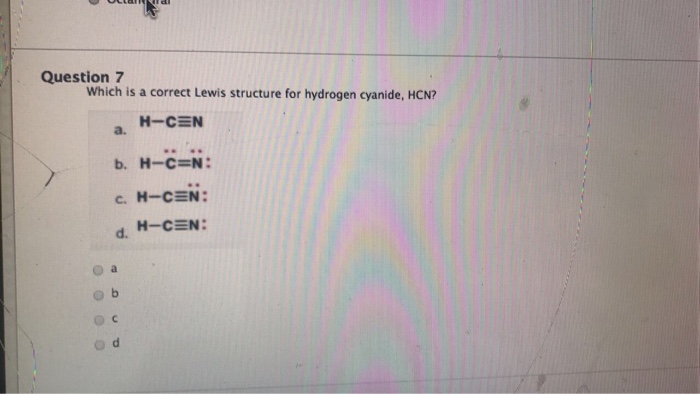
Solved Draw Charge Minimized Lewis Structures For The Following Compounds 2 Marks Each A Hcn B Course Hero
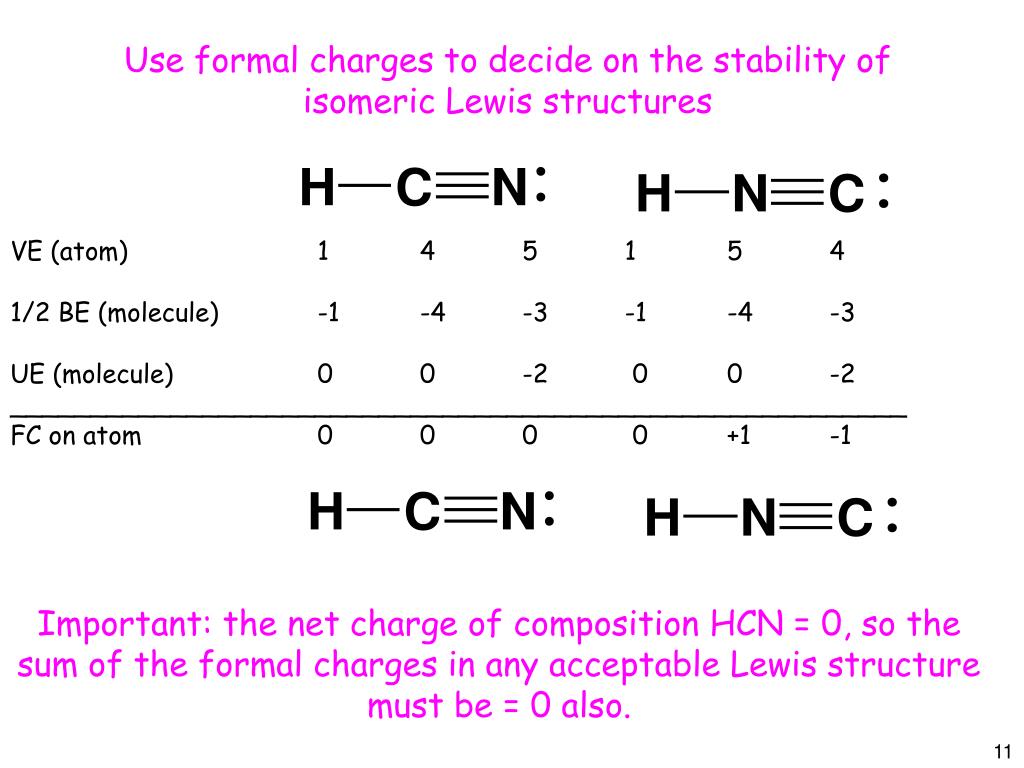
Ppt Chem C1403 Lecture 6 Lewis Structures And The Geometry Of Molecules With A Central Atom Powerpoint Presentation Id 1267874












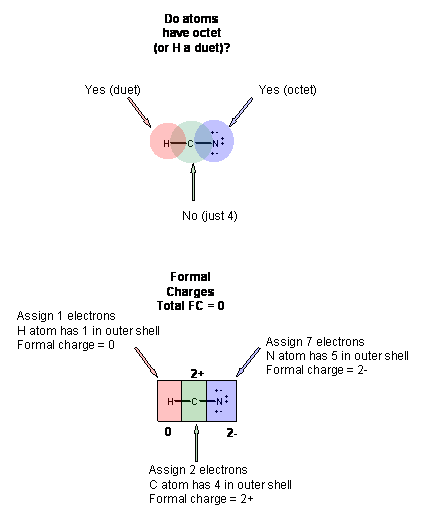








0 Response to "36 lewis dot diagram for hcn"
Post a Comment