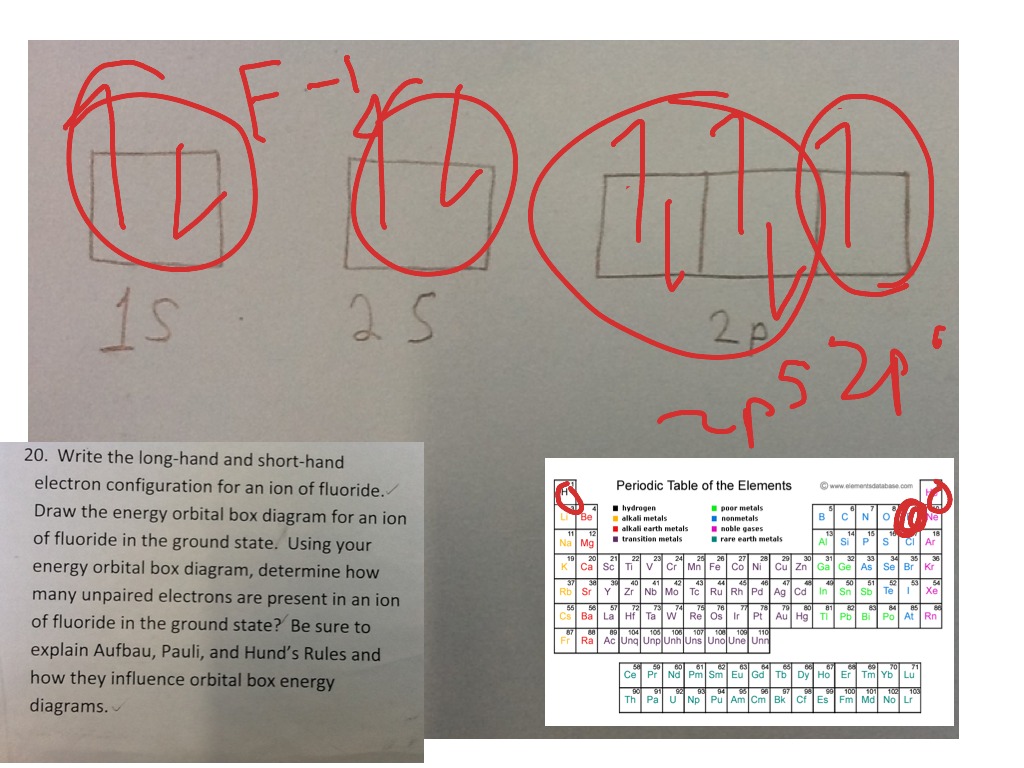
38 orbital diagram for f ion
Ion electron confugurat ion s. A neutral fluorine atom has 9 electrons. Show transcribed image text construct the orbital diagram of the f ion. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbital s in order of increasing energy, starting at the bottom with the. Answer to Write orbital diagram for Co2+. Answer: The correct ground state electron configuration of F− − ion is option c. 1s22s22p6. 1 s 2 2 s 2 2 p 6 . Explanation: The atomic number of fluorine ...
Construct the orbital Diagram Of the F- Ion. construct construct the orbital diagram the f- ion chem 120a november 8 2005 fall 2004 8 00 - 9 20 am exam ii name prepare a molecular orbital energy level diagram for no construct a solved construct the orbital diagram the f ion a ne answer to construct the orbital diagram of the f ion a neutral fluorine atom has 9 electrons how many ...

Orbital diagram for f ion
It is common to omit the core electrons from molecular orbital diagrams and configurations and include only the valence electrons. Figure 8. This is the molecular orbital diagram for the homonuclear diatomic Be 2 +, showing the molecular orbitals of the valence shell only. The molecular orbitals are filled in the same manner as atomic orbitals ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... ... forms stable ions that have incompletely ... A transition metal is defined as an element that forms a stable ion with incompletely filled d orbitals.
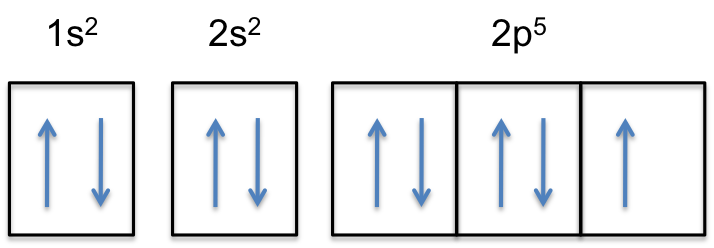
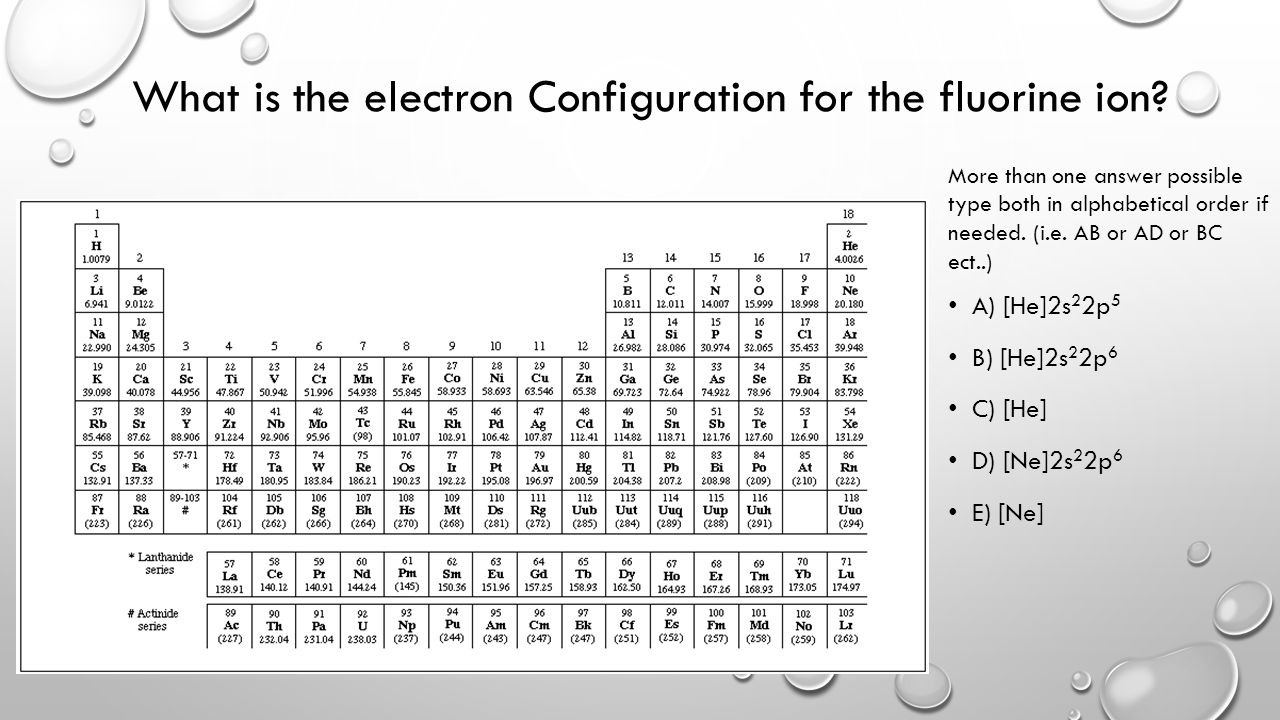
Orbital diagram for f ion. Choose the valence electron orbital diagram that represents the ground state of Se2-D (#1) The solid compound K2S2O3 contains. K+ ions and S2O3^2- ... The _____ is the change in energy associated with gaining an electron in the gaseous state for an atom or ion. electron affinity. Since electrons are negative, gaining one electron results in F having a 1- charge. To write the electron config all we have to do is add one ... ... first quarter of General Chemistry and covers the following topics: atomic structure; general properties of the ... Lecture 23 - Final Exam Review ";F"^(-): 1s^2 2s^2 2p^6 A good starting point for when you must find the electron configuration of an ion is the electron configuration of the neutral atom. In your case, you must find the electron configuration of the fluoride anion, ";F"^(-), so start by writing the electron configuration of a neutral fluorine atom, ";F". Fluorine is located in period 2, group 17 of the periodic table and has ...
Filled in: Oxygen Ion Dot Diagram For Oxygen Ion 9 out of 10 based on 70 ratings. ... Ions Are Created When Atoms Lose Or Gain ... Ion Formation Fluorine (F) Electron Configuration with Full Orbital Diagram. Fluorine electron configuration is 1s 2 2s 2 2p 5. The symbol for fluorine is F. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine (F) and the orbital diagram is the main topic of this article. The electronic configuration for the Fluorine ion is 1s22s22p5 and in this configuration, Fluorine needs 1 electron so as to complete the 2p ... Oct 29, 2019 - In this video we will write the electron configuration for F-, the Fluoride ion. We'll also look at why Fluorine forms a 1- ion and how the ...
Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is 'Na'. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles. ... the Chemistry 8 class page for ... Use of the page: Assignments are added from the bottom up. ... Comparison Physical Properties: The five categories Electron Configuration For Nitrogen Ion. The atomic number of nitrogen is 7, the element nitrogen was discovered by a Scottish physician, Danial Rutherford. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element Nitrogen. ... When we talk about the orbital ... Orbital diagram of f ion. The remaining five electrons will go in the 2p orbital. The following is the diagram for the neutral oxygen. Electron configurations orbital diagrams. An orbital diagram naturally leads to the writing of an electron configuration. Ion electron confugurations.
PT tells us how multiple electrons are filled in an atom ... I will give you five (5) species and you write the configurations
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
Build the orbital diagram for the ion most likely formed by phosphorus. Electron Configurations of Atoms and Ions. The electron configuration of an atom tells us how many electrons are in each orbital. For example, helium has two electrons in the 1s orbital. Therefore the electron configuration of He is 1s².
Build the orbital diagram for the ion most likely formed by phosphorus. ... what is the orbital diagram for Au+, how do you fit the f orbitals in?
Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.
Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic ...
Menu: Double right-click for options; for example, changing the orbital number, rendering or background ... Web Viewer (2 MB) is available free from ...
This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion ...
When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ...
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
(f) Use the pictured MOs to help you construct an MO energy level diagram for the valence π orbitals only of the carbonate ion. Remember to include the valence π electrons on your MO diagram.
Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ...
orbital energy-level diagram for the H2-ion. Bond order = ½ (2 - 1) = 0.5 Electron Configurations of Diatomic Molecules of the Second Period 1. Homonuclear diatomic molecules such as Li 2 utilize only F orbitals. For filled K shell bonding and antibonding orbitals use KK designation. 2.Be2 = KK(F2s)2(F2s*)2 Bond order = 1/2 (2-2) = 0 So Be2 ...
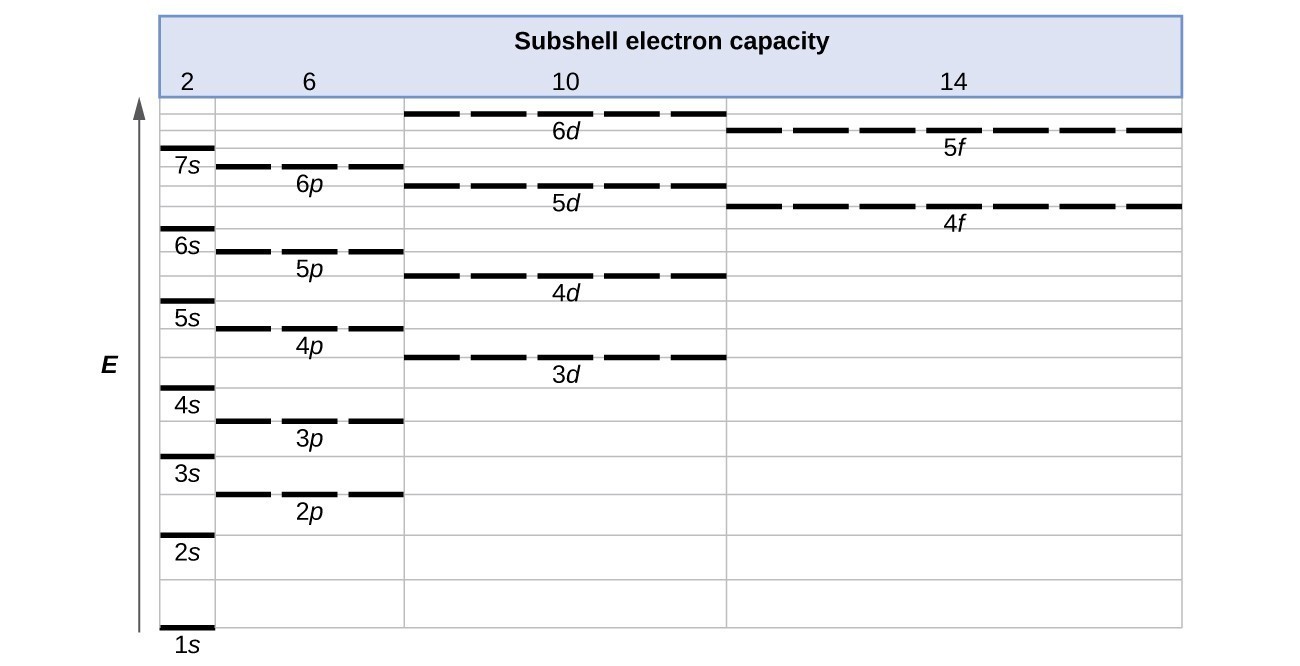
For example, the 5s orbital is of lower energy than the 4d orbital (see Figure 3 for a complete pattern of orbital levels). Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The orbital filling diagram of lithium. The electron configuration of lithium is 1s²2s¹.
Question: Construct The Orbital Diagram Of The F^- Ion. A Neutral Fluorine Atom Has 9 Electrons. How Many Electrons Does A F^- Ion Have? This problem has been solved! See the answer. Show transcribed image text. Videos. Step-by-step answer 05:59 0 0. Expert Answer 100% (22 ratings) ...

In The Ground State Electron Configuration Of Fe3 How Many Unpaired Electrons Are Present Woodworking
... Notes IC 1 4-5 Orbital Diagrams Notes IC 1 14 3-A: Orbital Diagrams Worksheet ... Orbital Diagrams & Electron Configurations for Atoms and Ions

Electron Shell Fluorine Atom Periodic Table Chemical Element Great Element Chemical Element Angle Smiley Png Pngwing
Compare the atomic and molecular orbital diagrams to identify the member of each of the following pairs that has the highest first ionization energy (the most tightly bound electron) in the gas phase: ... What charge would be needed on F 2 to generate an ion with a bond order of 2? Solution. 2+ Predict whether the MO diagram for S 2 would show ...
Answer (1 of 4): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...
F orbital Diagram. 1 4 electron configuration and orbital diagrams orbital diagrams many times it is necessary to see all the quantum numbers in an electron configuration this the purpose of the orbital diagram s p d f orbitals chemistry the orbital names s p d and f describe electron configuration these line groups are called sharp principal diffuse and fundamental the orbital
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
5.9 a. A diagram is sketched at the right. Since the difference in valence orbital potential energy between the 2s of N (-25.56 eV) and the 2p of F (-18.65 eV) is 6.91 eV, the 2p orbital is expected to be higher in energy relative to the degenerate 2p set. b. NF is isoelectronic (has the same number of valence electrons) with O2. Therefore, NF ...

1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2
Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H
The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
Problem: Construct the orbital diagram of the F- ion.A neutral fluorine atom has 9 electrons. How many electrons does a F- ion have? FREE Expert Solution. We are asked to construct the orbital diagram of the F-ion. F → 9 electrons. Negative charge adds 1 electron more. F-→ 10 electrons.
Orbital Diagram: Orbital diagram shows how electrons are distributed in various kinds of shells in the increasing order for a particular ion or element. The filling of electrons is studied and ...
F^- : 1s^2 2s^2 2p^6 alternatively: F^- : [Ne] Elemental Fluorine has an electron configuration of 1s^2 2s^2 2p^5 and needs 1 more electron ...
... forms stable ions that have incompletely ... A transition metal is defined as an element that forms a stable ion with incompletely filled d orbitals.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
It is common to omit the core electrons from molecular orbital diagrams and configurations and include only the valence electrons. Figure 8. This is the molecular orbital diagram for the homonuclear diatomic Be 2 +, showing the molecular orbitals of the valence shell only. The molecular orbitals are filled in the same manner as atomic orbitals ...

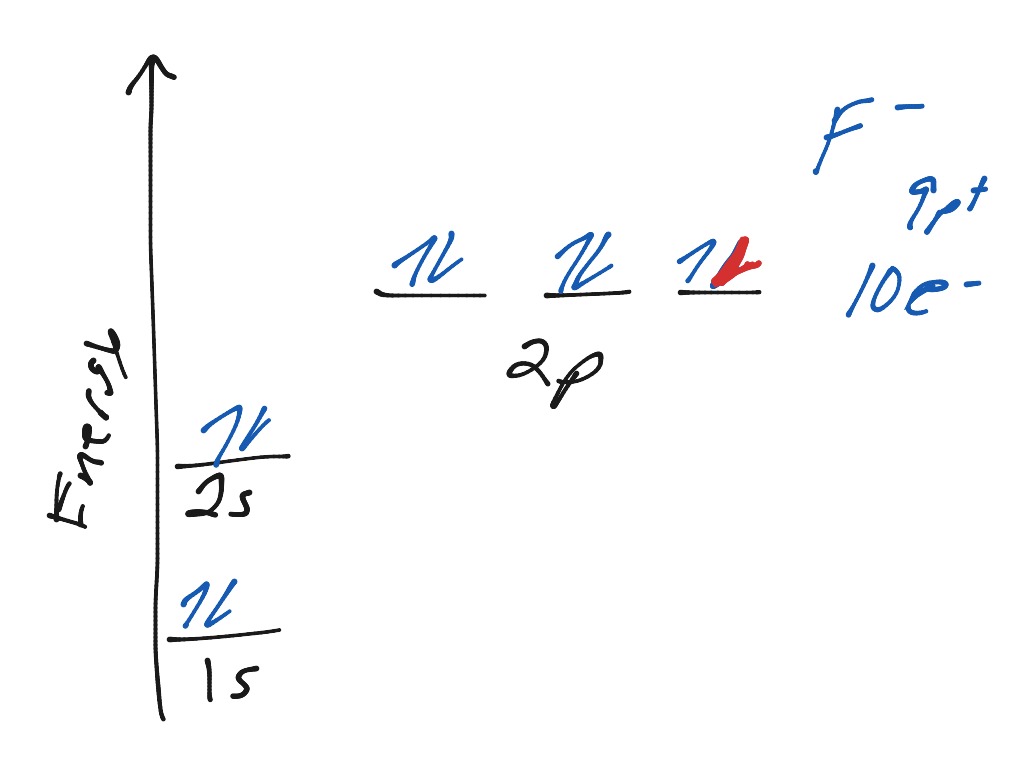











/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)






0 Response to "38 orbital diagram for f ion"
Post a Comment