39 d orbital energy level diagram
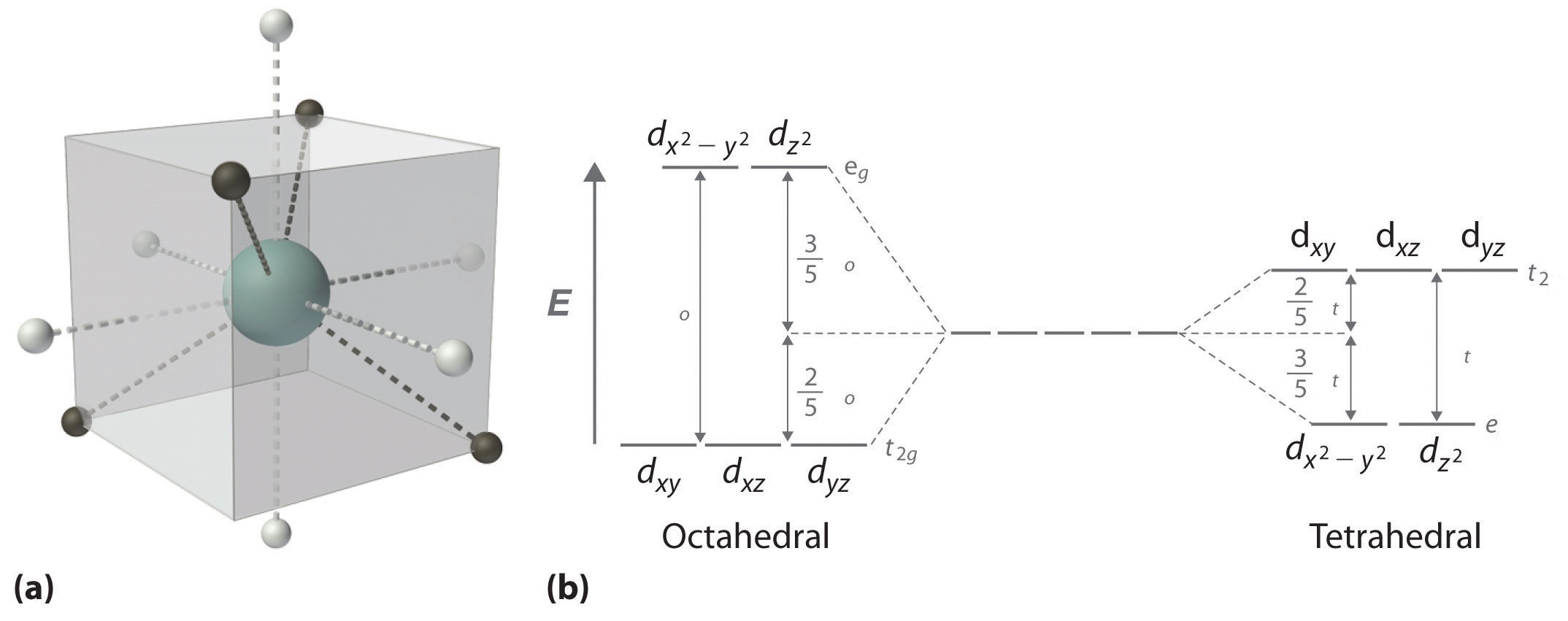
• The energy increase of the e g orbitals and the energy decrease of the t 2g orbitals must be balancedrelative to the energy of the hypotheticalsphericalfield(aka the barycenter).• The energy of each of the two orbitals of the e g set rises by +3/5 o (+6 Dq) while the energy of eachof the three t 2g orbitalsfallsby ‐2/5 o(‐4Dq). • Thisresults inno netenergy changefor the system: The splitting pattern of d-orbital energy levels of d1-metal complexes in trigonal distortion. Distortion in Four-Coordinated Complexes The tetra-coordination of ligands in different metal complexes can be categorized mainly as the tetrahedral and square planer. The tetrahedral geometry can be distorted in various ways to produce other four-
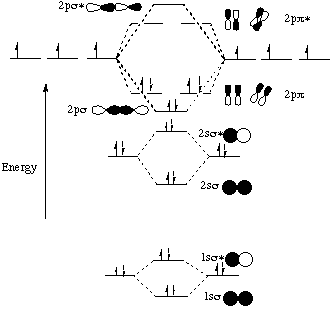
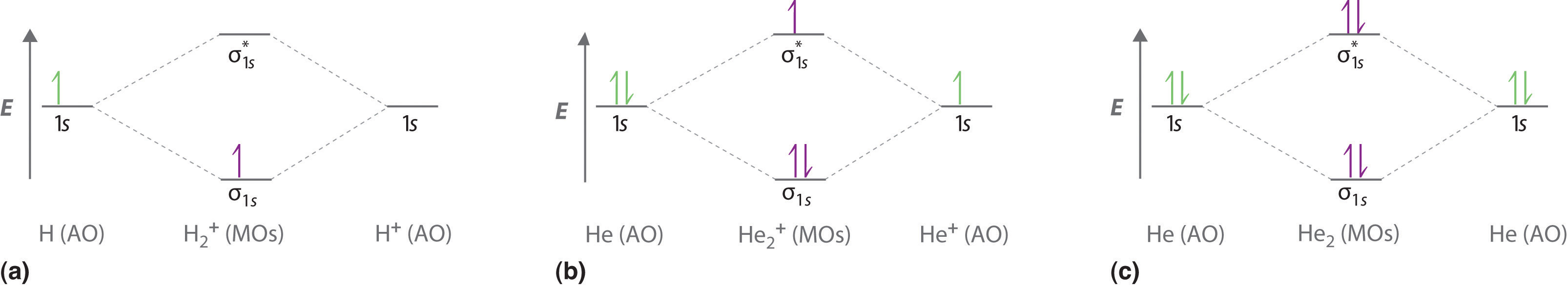
The molecular orbital energy- level diagram that results is constructed by putting the molecular orbitals in order of increasing number of internuclear nodal planes, the orbital with no such nodal plane lying at lowest energy and the orbital with nodal planes between all the atoms lying at highest energy. A molecular orbital diagram, or MO ...

D orbital energy level diagram
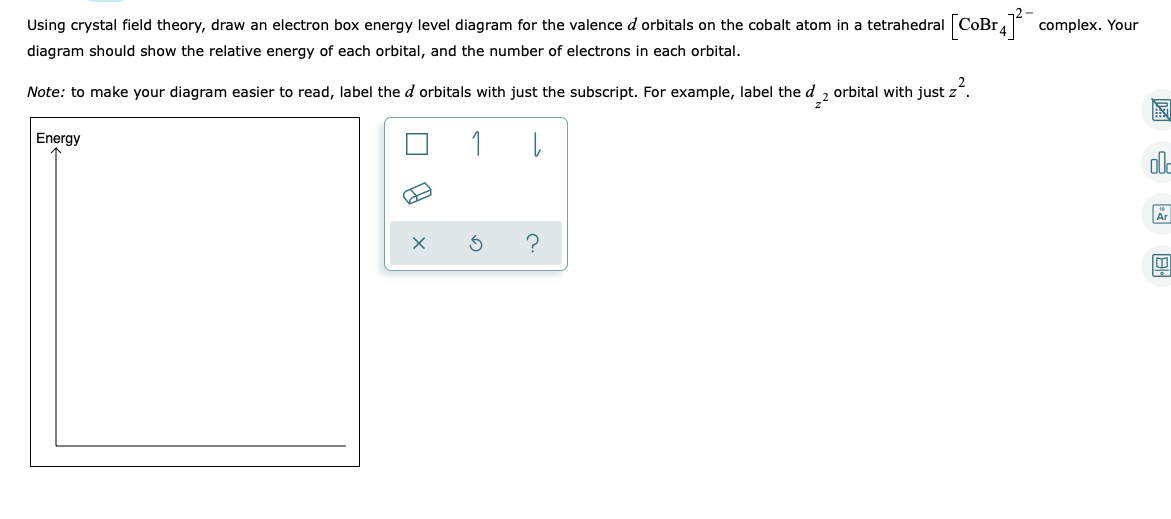
Stable electronic configurations: MO Energy Level Diagrams Reviewed ... Ligand (σ- and π*) orbitals and metal d-orbitals are shown.) Simplified MO energy ...37 pages A P-Orbital in the second energy level is a 2p orbital ( 2p(x), 2p(y), 2p(z) ) A P-Orbital in the third energy level is a 3p orbital ( 3p(x), 3p(y), 3p(z) ) etc. In addition, the third and subsequent energy levels each contain five D-Orbitals, the fourth and subsequent energy levels contain seven F-Orbitals and so on. Chemistry questions and answers. Using crystal field theory, draw an electron box energy level diagram for the valence d orbital on the cobalt atom in a [CoBr_6]^3- complex. Your diagram should show the relative energy of each orbital, and the number of electron in each orbital.
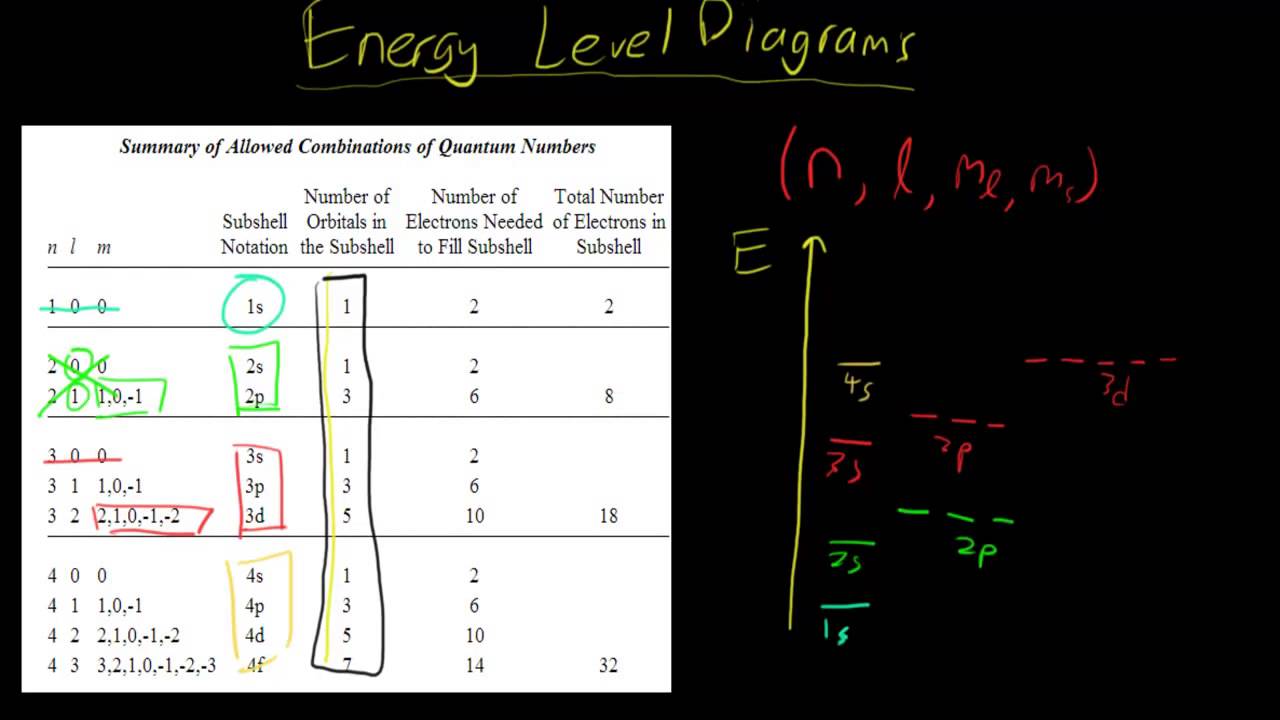
D orbital energy level diagram. • Energy Level -Called principal energy levels -Corresponds to row on periodic table -As n increases, E increases and the electron is ... Orbital diagram - orbital is a box grouped by sublevel containing arrow(s) to represent electrons Electron Configuration . Section 11.3 Atomic Orbitals The CFT diagram for tetrahedral complexes has d x 2 −y 2 and d z 2 orbitals equally low in energy because they are between the ligand axis and experience little repulsion. In square planar molecular geometry, a central atom is surrounded by constituent atoms, which form the corners of a square on the same plane. Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram . For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Energy level diagram for Molecular orbitals. ... If N b = Na,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals. 2) Stability of molecules in terms of bond order.
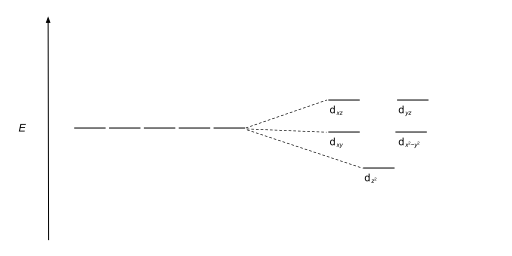
The octahedral ion [Fe(NO2)6]3−, which has 5 d-electrons, would have the octahedral splitting diagram shown at right with all five electrons in the t2g level.Overview of crystal field theory · Crystal field stabilization energy 11 Oct 2021 · 1 answer(a) The complex ion, [Ni(en)3]2+ is octahedral. Since en is a strong ligand there is pairing of electrons. Number of unpaired electrons = n ... Therefore, the electron energy levels of these five d-orbitals in mechanics forming a set five-fold degenerate energy level and the shape diagram dimensions of these orbitals identify by the principal, azimuthal, magnetic, spin quantum number or numbers of the atom for physics or chemistry courses. Explanation of Degenerate Orbitals with Diagram. Orbitals in the 2p sublevel are degenerate orbitals - Which means that the 2p x, 2p y, and 2p z orbitals have the exact same energy, as illustrated in the diagram provided below. Similarly, the 3p x, 3p y, and 3p z are degenerate orbitals. And at the 3d energy level, the 3d xy, 3d xz, 3d yz, 3d ...
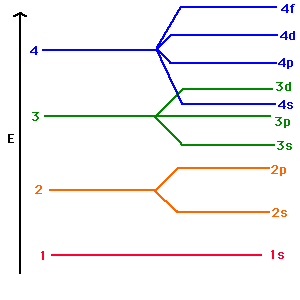
The Energy Level, known popularly as the electron shell, is the orbit with electrons that surround the nucleus of an atom. As per definition, the ascending order arrangement of energies in an orbital is called Energy Level Diagram. Figure 6.27 "Orbital Energy Level Diagram for the Hydrogen Atom" shows that the energy levels become closer and closer together as the value of n increases, as expected because of the 1/n 2 dependence of orbital energies. Figure 6.27 Orbital Energy Level Diagram for the Hydrogen Atom. The Aufbau principle tells you that the lowest-energy orbitals fill first, but the specific order isn't sequential in a way that's easy to memorize. See Resources for a diagram showing the filling order. Note that the n = 1 level only has s orbitals, the n = 2 level only has s and p orbitals, and the n = 3 level only has s, p and d orbitals. 9 May 2021 — The energy difference between the two d orbital levels is ... Like all ligand-metal interaction diagrams, the energy levels of the ligands ...
Molecular Orbital Theory: Energy level diagram for molecular orbitals. Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern and more rational. This theory assume that in molecules, atomic orbitals lose their identity and the electrons in molecules are present in new orbitals called molecular orbitals.

Scielo Brasil Explaining The Geometry Of Simple Molecules Using Molecular Orbital Energy Level Diagrams Built By Using Symmetry Principles Explaining The Geometry Of Simple Molecules Using Molecular Orbital Energy Level Diagrams Built
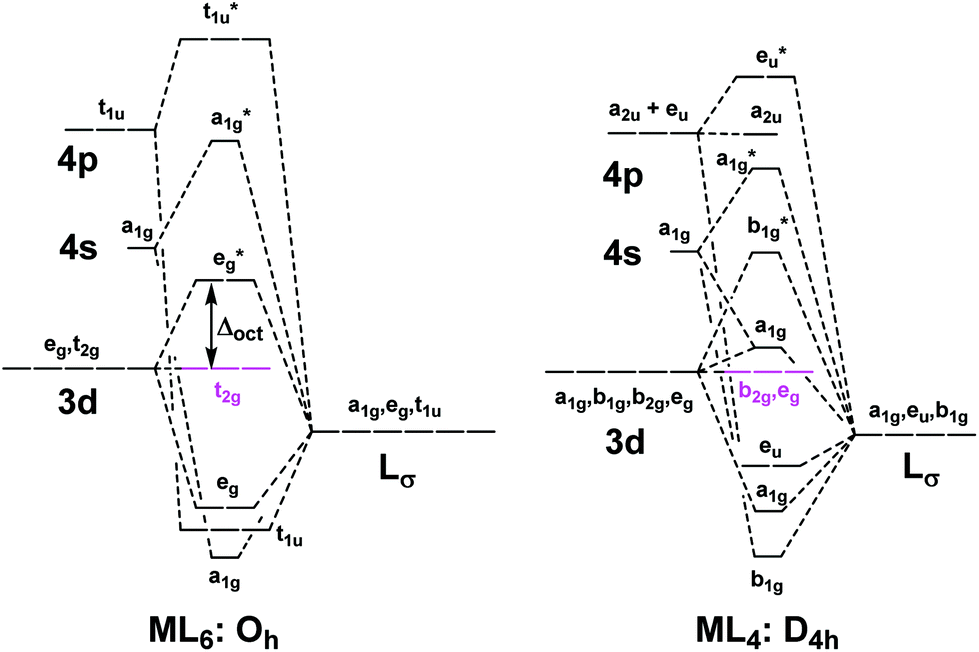
The overall molecular orbital energy level diagram for σ-bonding in octahedral complexes can be shown as: Figure 10. The formation of σ-molecular orbitals (bonding, antibonding and non-bonding) in octahedral complexes of transition metals. Buy the complete book with TOC navigation,
with the. d. orbital being one level lower than the energy level it is on. orbital diagram for arsenic see more ideas approximately from diagram electron configuration gallery & create your house design images related to pictures as well help you in locating the solution are seeking about itOrbital diagram for arsenic.
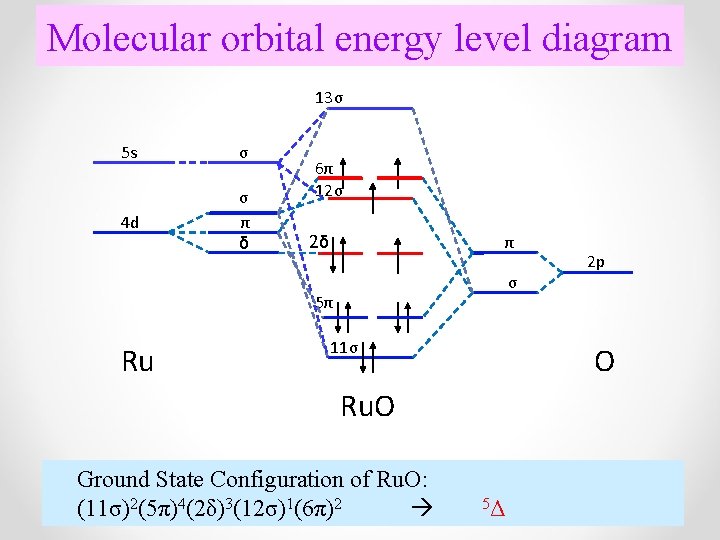
molecular orbital energy-level diagram for the NO molecule. We assume that orbital order is the same as that for N2. The bond order is 2.5. Figure 9.42: The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure 9.43: A partial molecular orbital energy-level diagram for the HF molecule.
The energy of an orbital depends on the shape and size of the orbital. In a multiple-electron system, the shielding effect also influences the orbital's energy. Energies of orbitals are quantized as per quantum mechanics. Thus, there are only selected energy levels available, which an electron can occupy. An electron residing in a particular ...
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
OOPS!! "Where an atom's ELECTRONS go" is what the heading should read.Electron orbital diagrams showing energy levels (n), subshells or sublevels(s, p, d, f...
A molecular orbital is a region of space in a covalent species where electrons are likely to be found. The combination of two atomic orbitals always forms two molecular orbitals; the bonding molecular orbital, which is _____ in energy, and the antibonding molecular orbital, which is _____ in energy, than the original atomic orbitals.
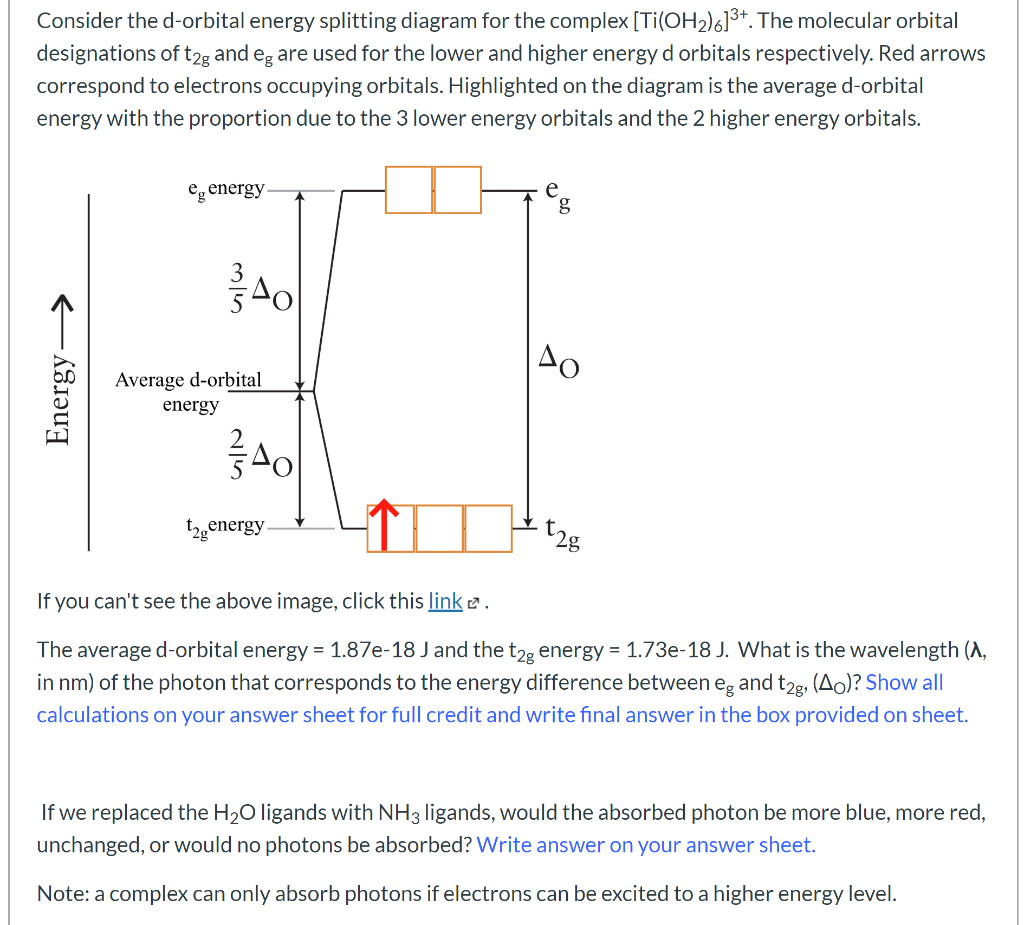
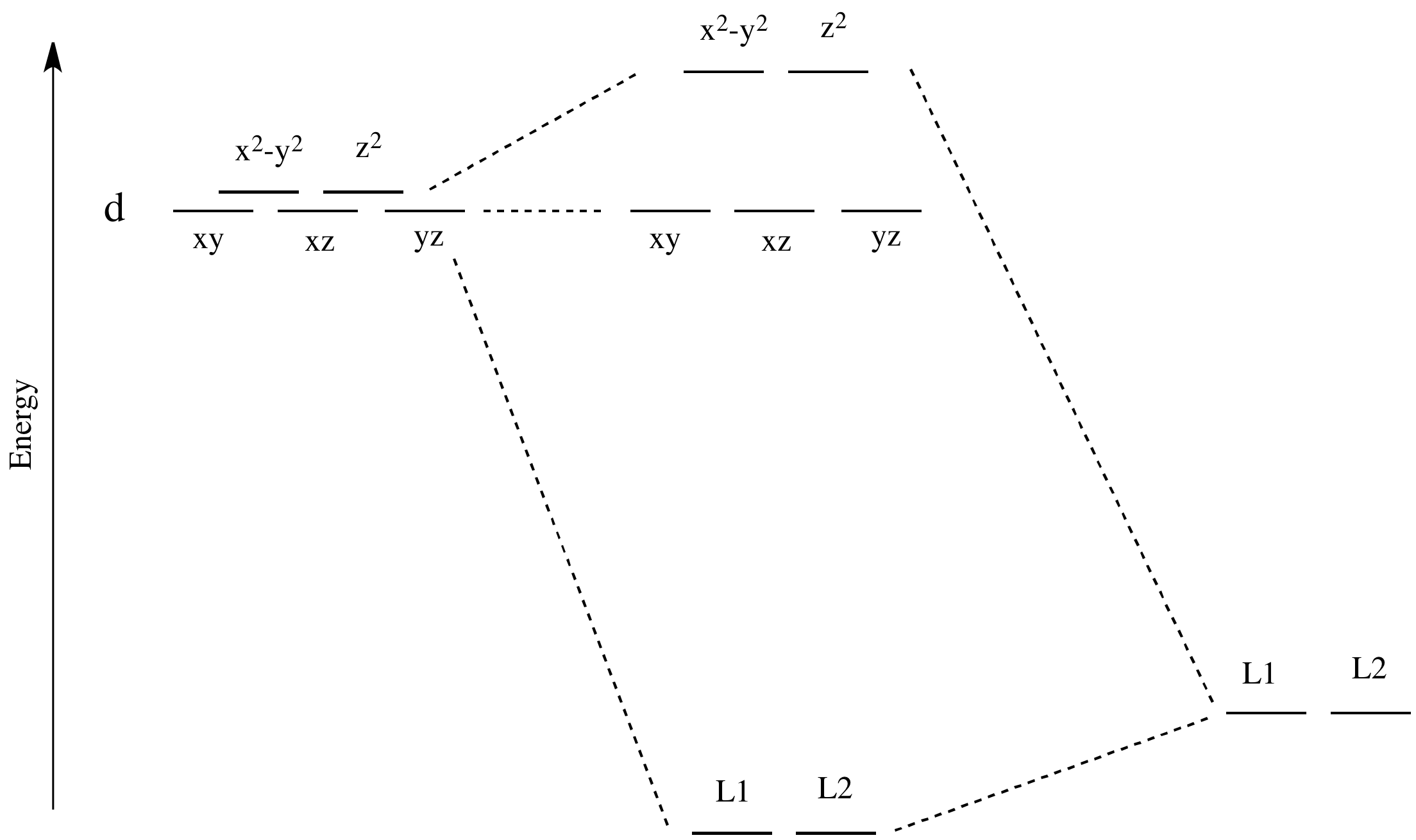
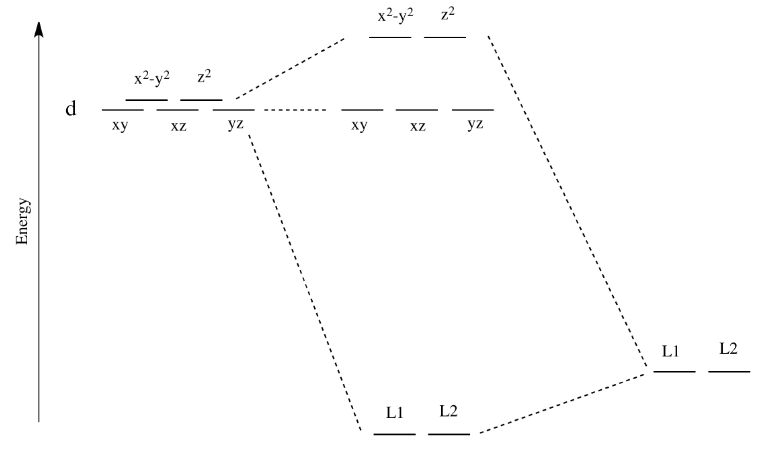
Like all ligand-metal interaction diagrams, the energy levels of the ligands by themselves are shown on one side. The metal's electronic energy levels are shown on the other side. The result of their interaction, a metal-ligand complex, is shown in the middle. The d orbital splitting diagram is shown in a box.
Energy Levels, Orbital Diagrams, Electron Config, Noble Gas - Google Slides. Energy Levels. We know, atoms have energy levels. The periodic table shows us energy levels 1 - 7. Energy Levels. By default electrons are found in the lowest energy level possible, close to the nucleus. This is called the ground state.
Answer to: Draw an orbital energy-level diagram showing the configuration of the d-electrons on the metal ion in the complex NiCl42- (tetrahedral)....1 answer · Top answer: The given coordination complex ion is [NiCl4]2−[NiCl4]2−. The central metal atom in {eq}{\left[...
Molecular Orbital Energy Level Diagrams: Linear Polyatomic Molecules . This exercise assumes that you are familiar with the "count and sort" algorithm described in Exercise 2.3 (which also contains a table of atomic orbital energies).
All levels except the first have p orbitals. d ORBITALS. In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels. At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3px, 3py, 3pz). At ...
This video explains s, p, d, and f orbitals, sublevels, and their shapes. It discusses the 4 quantum numbers n, l, ml, and ms. n represents the energy leve...
The molecular, sp 3 orbitals are arranged in a tetrahedron, with bond angles of 109.5 o. Each of the 1s orbitals of H will overlap with one of these hybrid orbitals to give the predicted tetrahedral geometry and shape of methane, CH 4. Hybridization also changes the energy levels of the orbitals. The 2s orbital of carbon is lower in energy than the 2p orbitals, since it is more penetrating.
Recapitulation: Ligand field Theory: Octahedral Complexes ... as they lead to a splitting of the energy levels which are on the same order of magnitude.32 pages
Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral.1 page
Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿ ↿ ↿ ↿ 4s ↿⇂ 4p 4d 4f: ... Two of these are in the lowest energy level, eight are in the second energy level and then one electron is in the third energy level.
Download scientific diagram | Energy level ordering of d -orbitals in (a) tetrahedral and (b) octahedral crystal field environments. from publication: ...
Chemistry questions and answers. Using crystal field theory, draw an electron box energy level diagram for the valence d orbital on the cobalt atom in a [CoBr_6]^3- complex. Your diagram should show the relative energy of each orbital, and the number of electron in each orbital.
A P-Orbital in the second energy level is a 2p orbital ( 2p(x), 2p(y), 2p(z) ) A P-Orbital in the third energy level is a 3p orbital ( 3p(x), 3p(y), 3p(z) ) etc. In addition, the third and subsequent energy levels each contain five D-Orbitals, the fourth and subsequent energy levels contain seven F-Orbitals and so on.
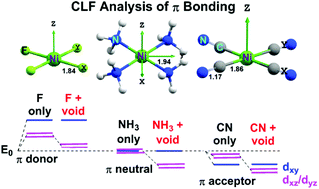
D Orbital Energy Levels In Planar Miif4 2 Mii Nh3 4 2 And Mii Cn 4 2 Complexes The Nature Of M L P Bonding And The Implications For Ligand Field Theory Dalton Transactions Rsc Publishing
Stable electronic configurations: MO Energy Level Diagrams Reviewed ... Ligand (σ- and π*) orbitals and metal d-orbitals are shown.) Simplified MO energy ...37 pages
D Orbital Energy Levels In Planar M Ii F 4 2 M Ii Nh 3 4 2 And M Ii Cn 4 2 Complexes The Nature Of M L

Scielo Brasil A Brief Introduction To Molecular Orbital Theory Of Simple Polyatomic Molecules For Undergraduate Chemistry Students A Brief Introduction To Molecular Orbital Theory Of Simple Polyatomic Molecules For Undergraduate














0 Response to "39 d orbital energy level diagram"
Post a Comment