36 copper-gold phase diagram
Generate Phase Diagram Compositional Phase Diagram; Aqueous Stability (Pourbaix) Tags: Gold cupride (1/1) Copper gold (1/1) - L1o type Tetraauricupride Gold copper (1/1) Material Details; Final Magnetic Moment 0.001 μ B. Calculated total magnetic moment for the unit cell within the magnetic ordering provided (see below). ... Copper in Powder Metallurgy. A. Phase Diagrams 1. Iron-Copper System The iron-copper phase diagram, taken from Hansen,3 is presented in Figure 1. Hansen3 also gives a thorough review of the work done on the system up to 1957. A review of more recent work, up to 1963 was done by . Elliott~ The most recent version of the phase diagram
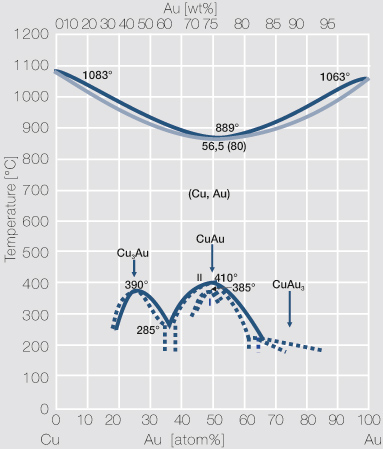
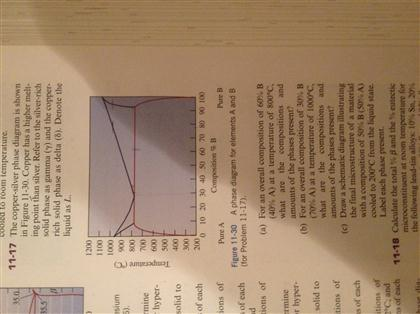
Solution The copper-gold phase diagram is constructed below. 9.10 Cite the phases that are present and the phase compositions for the following alloys: (a) 15 wt% Sn–85 wt% Pb at 100°C (212°F) (b) 25 wt% Pb–75 wt% Mg at 425°C (800°F) (c) 85 wt% Ag–15 wt% Cu at 800°C (1470°F)
Copper-gold phase diagram
Metals like silver and gold have a difference of 0.2%; nickel and copper of 2.7%, and show complete solid solubility. But zinc and copper have 4.2% difference with maximum solubility of 38.4 wt.% Zn. (other factors are less favourable); Cadmium in copper with 16.5% size difference shows a solid solubility of 1.7 wt.%. 10.4 Given here are the solidus and liquidus temperatures for the copper–gold system. Construct the phase diagram for this system and label each region. Composition Solidus Liquidus (wt% Au) Temperature (°C) Temperature (°C) 0 1085 1085. 20 1019 1042. 40 972 996. 60 934 946. 80 911 911. 90 928 942. 95 974 984. 100 1064 1064. Solution The ... Transcribed image text: Given below are the solidus and liquidus temperatures for the copper-gold system. Construct the phase diagram (using MATLAB or Excel) for this system and label each region. Composition (wt % Au) 0 20 40 60 80 90 95 100 Solidus Temperature (degC) 1085 1019 972 934 911 928 974 1064 Liquidus Temperature (degC) 1085 1042 996 949 911 942 984 1064
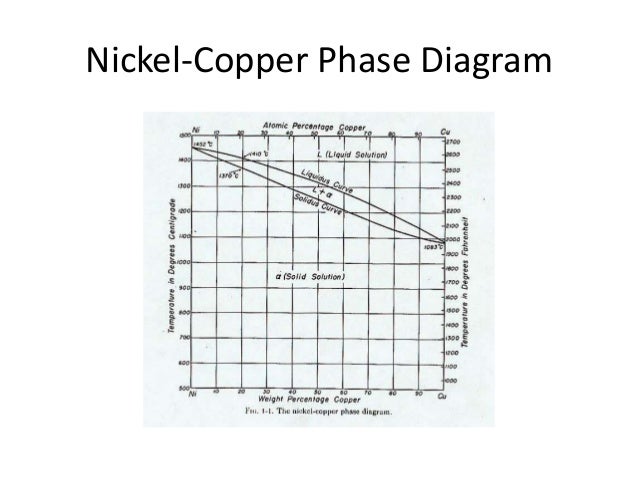
Copper-gold phase diagram. copper oxide is painted on the article and then gold balls are attached. The article is then placed in a reducing flame which causes the oxide to reduce to copper and then react and form a eutectic with the gold which disappears by diffusion. Several objects that are thought to have been bonded using this process peritectics, and congruent phase transformations for the tin-gold system (Figure 9.36). There are two eutectics on this phase diagram. One exists at 10 wt% Au-90 wt% Sn and 217°C. The reaction upon cooling is L → α + β The other eutectic exists at 80 wt% Au-20 wt% Sn and 280°C. This reaction upon cooling is L → δ + ζ Use the following silver-copper phase diagram for Problems 5-9. 5. What are the solubility limits of Ag in (Cu) and Cu in (Ag)? Recall that (Cu) and (Ag) are the same as α and β, respectively. The solubility limit of Ag in (Cu) is 7.9 wt. % Ag. The solubility limit of Cu in (Ag) is 8.8 wt.% Cu. Note that these Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
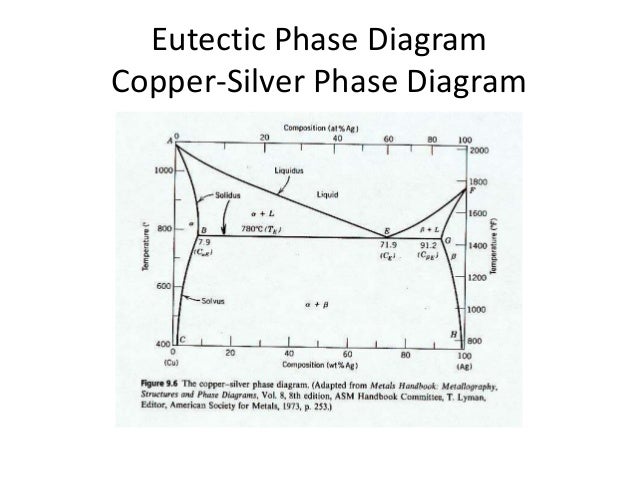
This representation is called a phase diagram. The phase diagrams of some binary systems relevant to bronze show the behavior of alloying elements that typically results in one of the three cases mentioned previously. The copper-tin equilibrium phase diagram (Figure 3) illustrates Cases (1) and (2). Sep 05, 2019 · Copper Silver Phase Diagram Phase Equilibria In The Agcllncl3 Ln Ce Nd Sm Gd Binary. Copper Silver Phase Diagram Colored Gold Wikipedia. Copper Silver Phase Diagram Solved 7 Consider The Binary Eutectic Copper Silver Phas. Copper Silver Phase Diagram Ppt Phase Diagrams Powerpoint Presentation Id230040 Construct the phase diagram for this system and label each region. (You can use excel or matlab to plot your phase diagram) Question: Given here are the solidus and liquidus temperatures for the copper gold system. Construct the phase diagram for this system and label each region. (You can use excel or matlab to plot your phase diagram) Phase diagram of gold-copper: You cannot overwrite this file. File usage. The following 2 pages link to this file: Gold Based Materials; Werkstoffe auf Gold-Basis; Metadata. This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original ...
The problem with phase diagrams is they become complicated with more than a base metal and one alloy. Figure 1shows a typical silver-copper phase diagram and it tells you a number of things. First, at all temperatures above the liquid line, any combination of silver and copper is liquid. peratures for the copper—gold system. Con- struct the phase diagram for this system and label each region. (h) 4.2 mol Cu and 1.1 mol Ag at 9000C (16500F) 9.9 Is it possible to have a copper—silver alloy that, sition 92 wt% Ag—8 wt% Cu, and also a liquid phase of composition 76 wt% Ag—24 wt% Cu? If so, what will be the approximate ... Reasonable and competitive prices for Phase Diagrams Of Binary Copper Alloys (Monograph Series On Alloy Phase Diagrams)|P our premium writing, formatting, editing and proofreading services;; Thorough, revolutionary and in-depth research, no matter the complexity of the work ordered; Plagiarism-free pieces of writing, as well as free plagiarism reports; Teach Yourself Phase Diagrams A.4 HRS 03/11/2009 and Phase Transformations PART 1: Key terminology Alloys and Components DEF.A metallic alloy is a mixture of a metal with other metals or non-metals. Ceramics too can be mixed
Gold-copper nano-alloy, "Tumbaga", in the era of nano: phase diagram and segregation Nano Lett. , 14 ( 11 ) ( 2014 ) , pp. 6718 - 6726 , 10.1021/nl503584q CrossRef View Record in Scopus Google Scholar
ในระบบที่ประกอบด้วยโลหะ 2 ชนิด (Binary phase diagram) ที่มีการ ... 12 Figure 9.2 The copper-nickel phase diagram . 3. ปริมาณหรือสัดส่วนของ Phase ที่ปรากฏอยู่ (Phase Amounts) 2.
The Au-Al phase diagram (Figure 14) contains a number of intermetallic compounds formed at compositions situated close to the gold-rich end of the diagram. AuAl2 has a gold content close to that of an 18 carat alloy (75 per cent gold), which makes it possible to produce a hallmarkable 18 carat purple alloy.
the thermal equilibrium diagram for the alloy of Copper and Nickel. In order to find what temperature 60% copper solidifies at we simply draw a vertical line from 60% copper until it hits the solidus line and at this is the point where 60% Copper has fully solidified. 0 100 10 90 20 80 30 70 40 60 50 50 60 40 70 30 80 20 90 10 100 0 900 1000 ...
Compositional Phase Diagram; Aqueous Stability (Pourbaix) Tags: Gold copper (1/3) Gold cupride (1/3) - HT Copper gold (3/1) Material Details; Final Magnetic Moment 0.004 μ B. Calculated total magnetic moment for the unit cell within the magnetic ordering provided (see below). ...
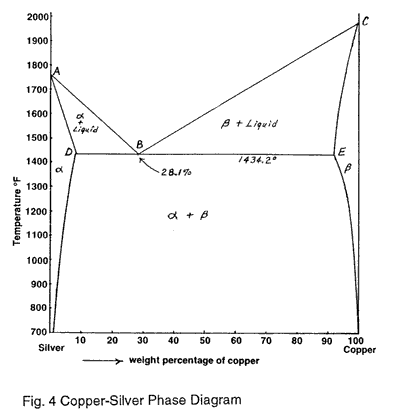
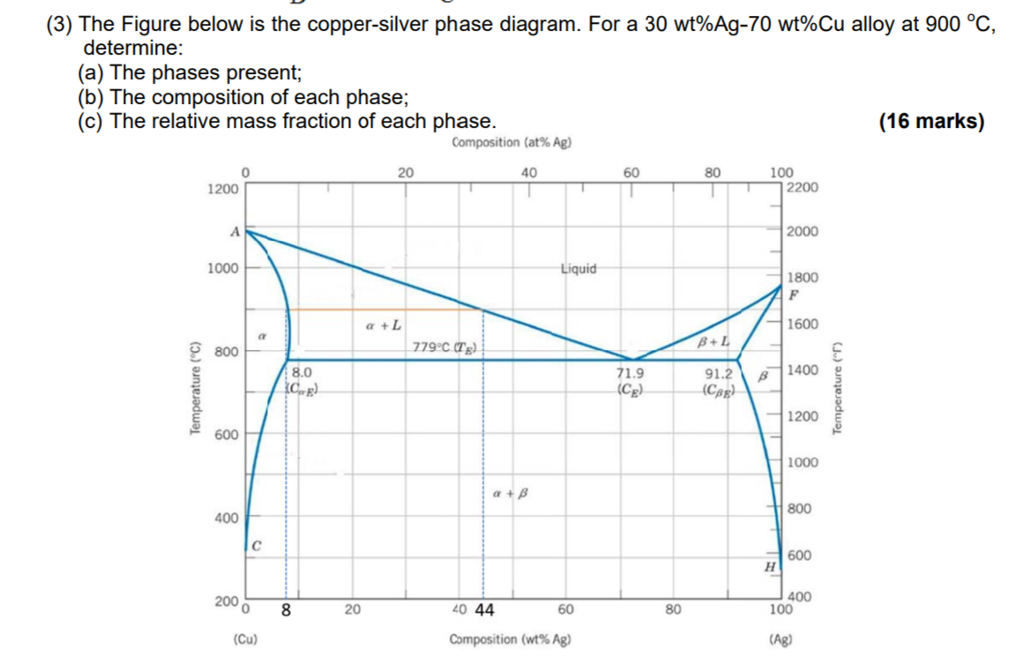
Download scientific diagram | Cu-Ag Phase Diagram. The eutectic composition is 28.1 wt% Cu-71.9 wt% Ag and the solid solubility limit of silver in copper is 8 wt% Ag (after Hansen and Anderko 1958 ...
phase diagrams. Copper-gold, which forms a continuous range of fcc solid solutions at elevated temperatures, has been exploited since ancient times [6] due to its natural occurrence and attractive reddish colour. Its binary phase diagram has been intensively investigated, primarily because its high temperature α-(Au,Cu) solid solution undergoes
All data taken from: "Binary Alloy Phase Diagrams", 2nd Edn., eds. T. B. Massalski, H. Okamoto, P. R. Subramanian and L. Kacprzak, in 3 volumes, ASM International, Ohio, USA, 1990 Temperature, ºC 2600 2200 1800 1400 1000 600 2447ºC 1769ºC 0 10 20 30 40 50 60 70 80 90 100 Ir Platinum, wt% Pt Platinum, at% 0 10 20 30 40 50 60 70 80 90 100 ...
Jun 24, 2016 · Abstract. Phase equilibria have been extrapolated to low temperatures, and a condensed phase diagram has been plotted for the Au–Cu system to be consistent with the third law of thermodynamics. Download to read the full article text.
Journal of Phase Equilibria - Indicates key paper06Rue: R. Ruer, "Alloys of Palladium with Gold,"Z. Anorg.Chem., 51, 391-396 (1906) in German.(Equi Diagram; Experimental; Indicates presence of a phase diagram)
Eutectic phase diagram for a silver-copper system. 2800 2600 2400 2200 2000 1800 1600 MgO CaO 20 40 60 80 100 0 C) L MgO ss + L MgO ss CaO ss + L CaO ss MgO ss + CaO ss Wt % Eutetic phase diagram for MgO-CaO system. Temperature (Lecture 19 – Binary phase diagrams 4 of 16 11/23/05
Transcribed image text: Given below are the solidus and liquidus temperatures for the copper-gold system. Construct the phase diagram (using MATLAB or Excel) for this system and label each region. Composition (wt % Au) 0 20 40 60 80 90 95 100 Solidus Temperature (degC) 1085 1019 972 934 911 928 974 1064 Liquidus Temperature (degC) 1085 1042 996 949 911 942 984 1064
10.4 Given here are the solidus and liquidus temperatures for the copper–gold system. Construct the phase diagram for this system and label each region. Composition Solidus Liquidus (wt% Au) Temperature (°C) Temperature (°C) 0 1085 1085. 20 1019 1042. 40 972 996. 60 934 946. 80 911 911. 90 928 942. 95 974 984. 100 1064 1064. Solution The ...
Metals like silver and gold have a difference of 0.2%; nickel and copper of 2.7%, and show complete solid solubility. But zinc and copper have 4.2% difference with maximum solubility of 38.4 wt.% Zn. (other factors are less favourable); Cadmium in copper with 16.5% size difference shows a solid solubility of 1.7 wt.%.


![Tin-Gold phase diagram [10] | Download Scientific Diagram](https://www.researchgate.net/profile/AM_Shkel/publication/224636210/figure/download/fig1/AS:645049176043525@1530803116954/Tin-Gold-phase-diagram-10.png)



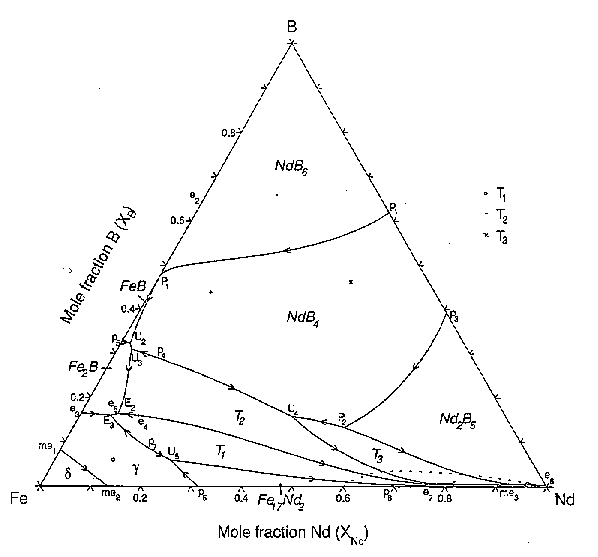







0 Response to "36 copper-gold phase diagram"
Post a Comment