36 the following lewis diagram represents the valence electron configuration of a main-group element.
Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. If this element is in period 6, ... •In a Lewis structure, we represent the valence electrons of main-group elements as dots surrounding the symbol for the element. -Also known as electron dot structures •We use the symbol of the element to represent the nucleus and inner electrons. Lewis Structures of Atoms
The transfer of this electron produces the Cs + ion, which has the valence electron configuration of Xe, and the F − ion, which has a total of eight valence electrons (an octet) and the Ne electron configuration. This description is consistent with the statement that among the main group elements, ions in simple binary ionic compounds generally have the electron configurations of the nearest ...
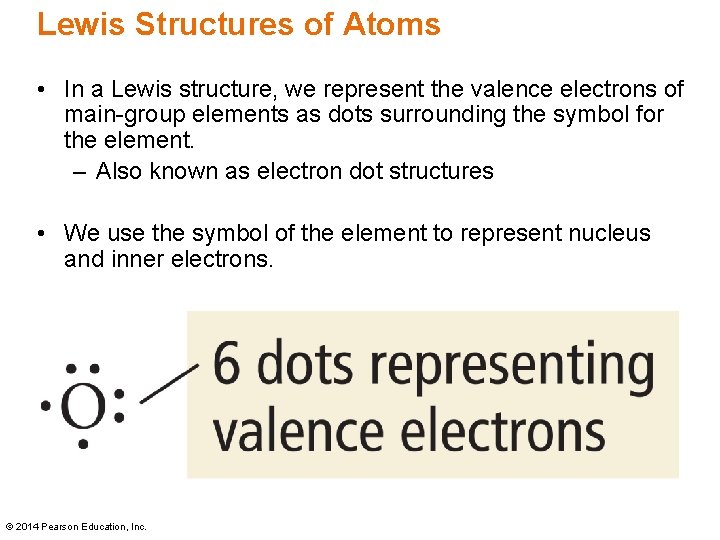
The following lewis diagram represents the valence electron configuration of a main-group element.
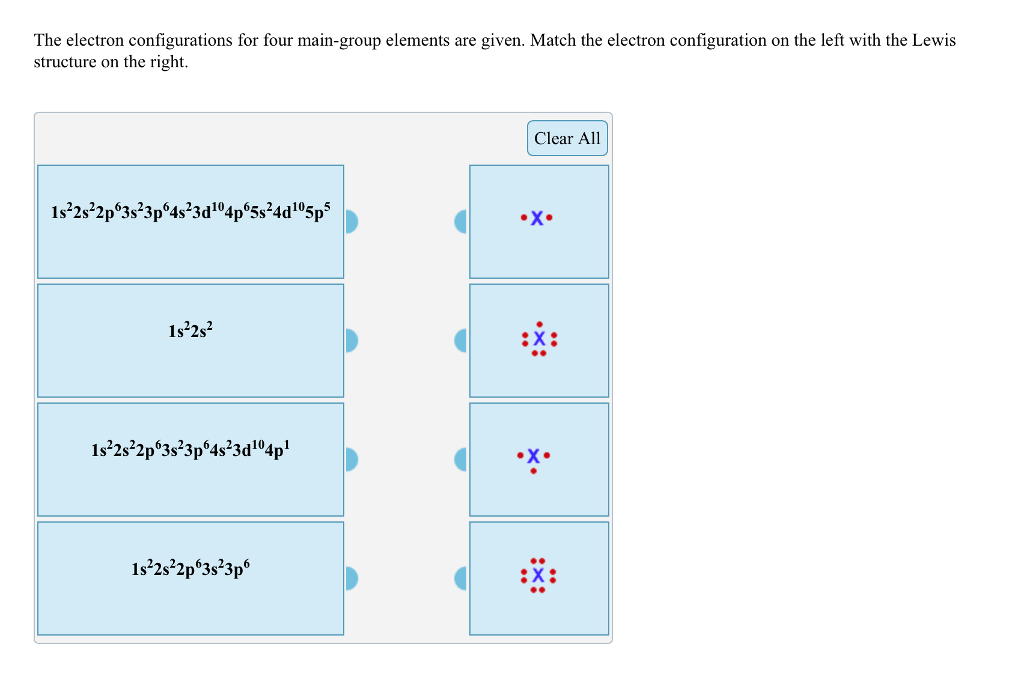
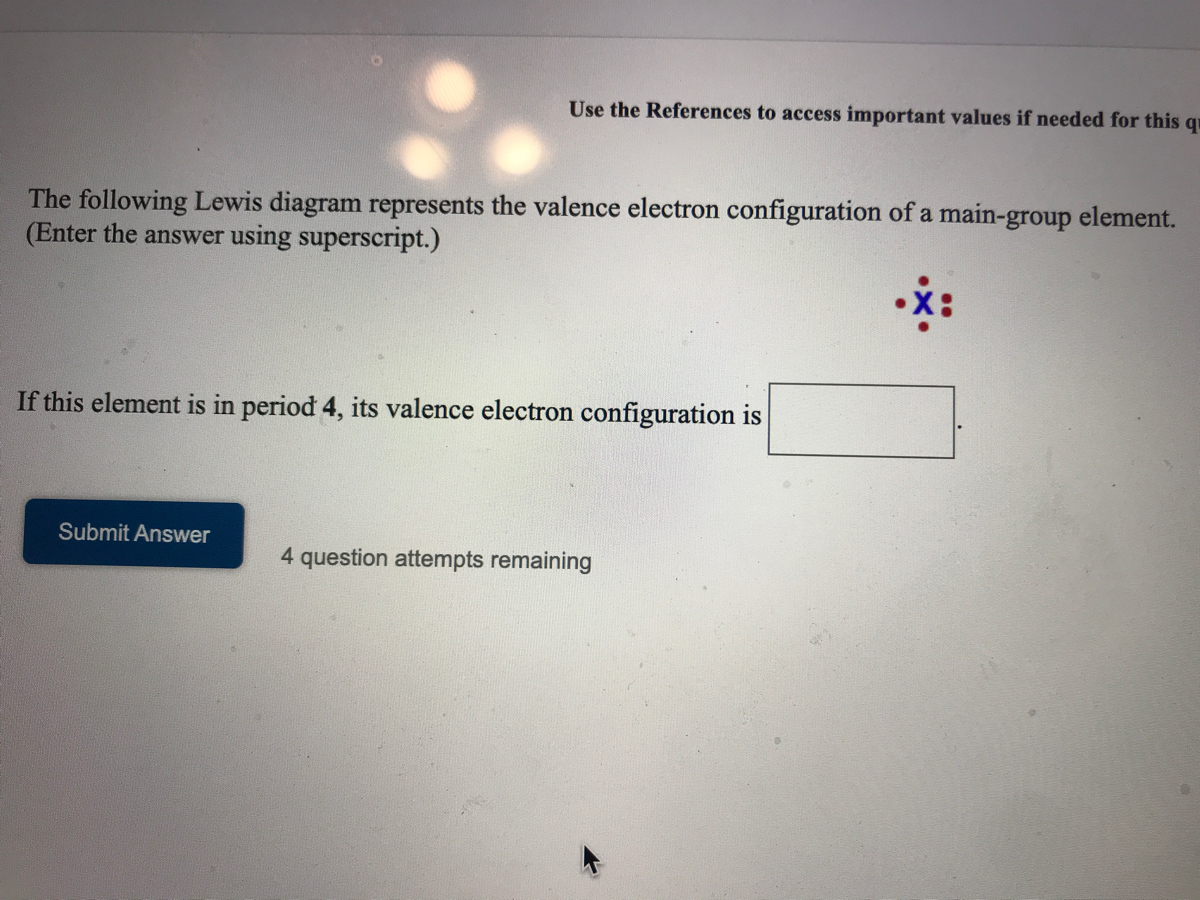
A main group element with the valence electron configuration 5s25p4 is in periodic group 16. It forms a monatomic ion with a charge of-2.B. A main group element with the valence electron configuration 5s1 is in periodic group 1. It forms a monatomic ion with a charge of +1.. The periodic group refers to the column of elements that are in the periodic table. ... We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1. Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. If this element is in period 4, ...
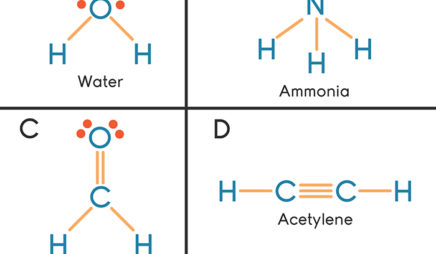
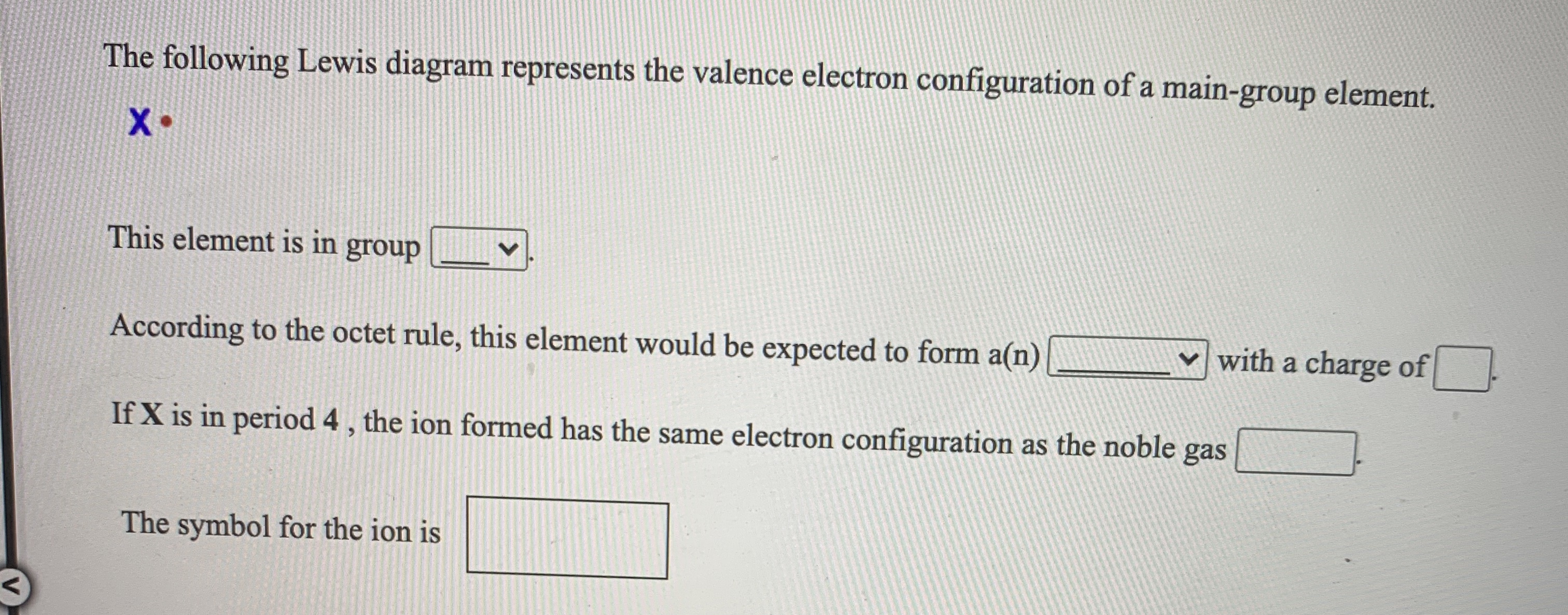
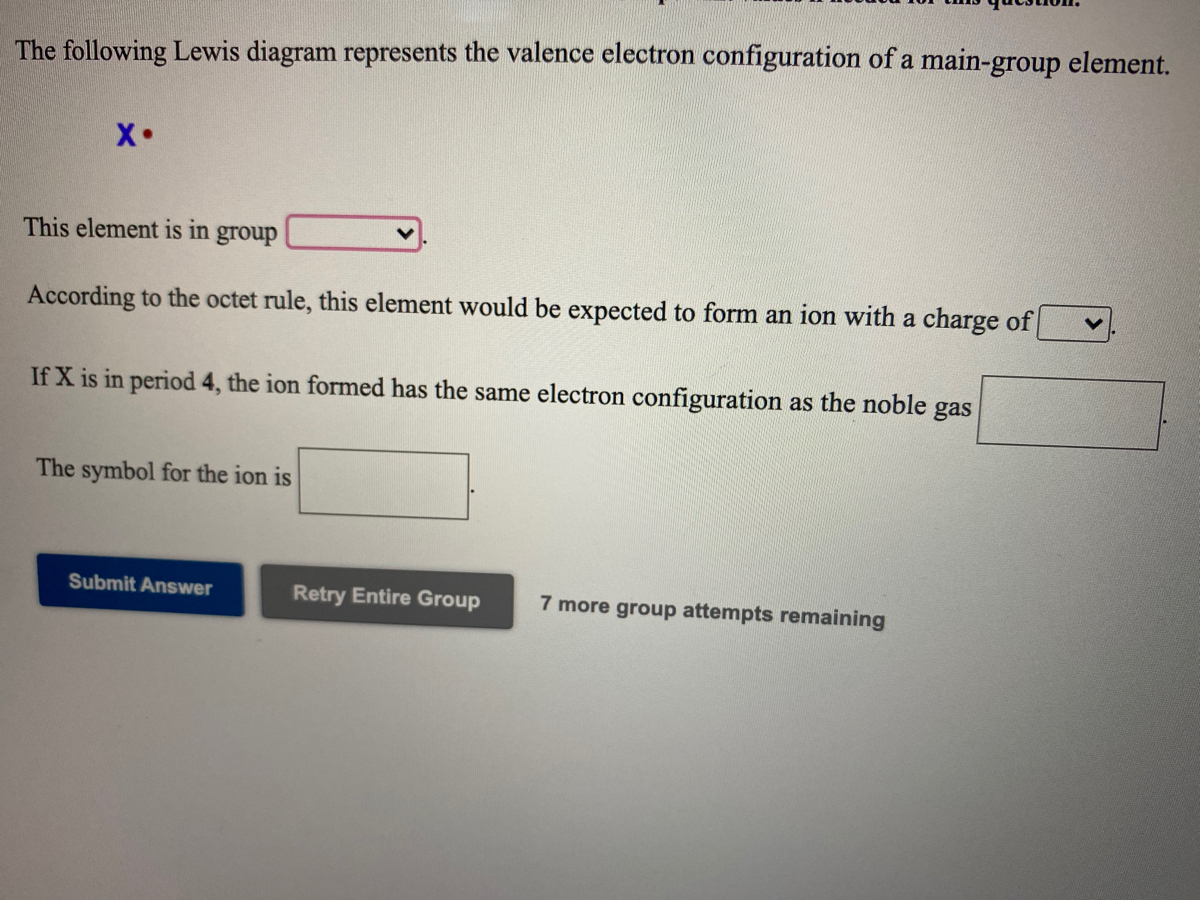
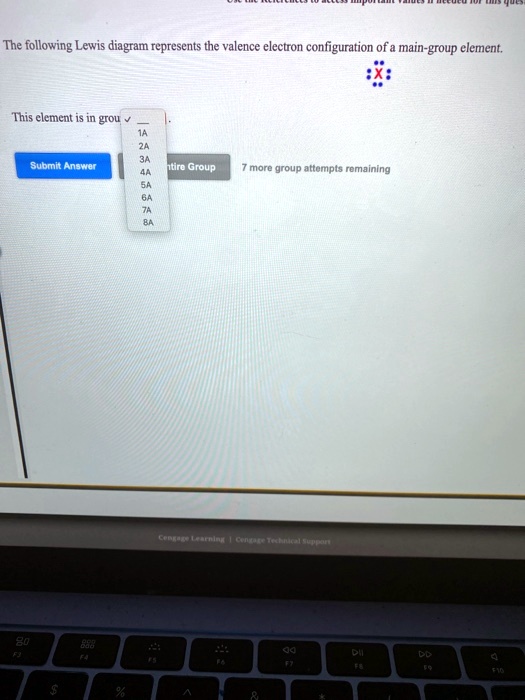
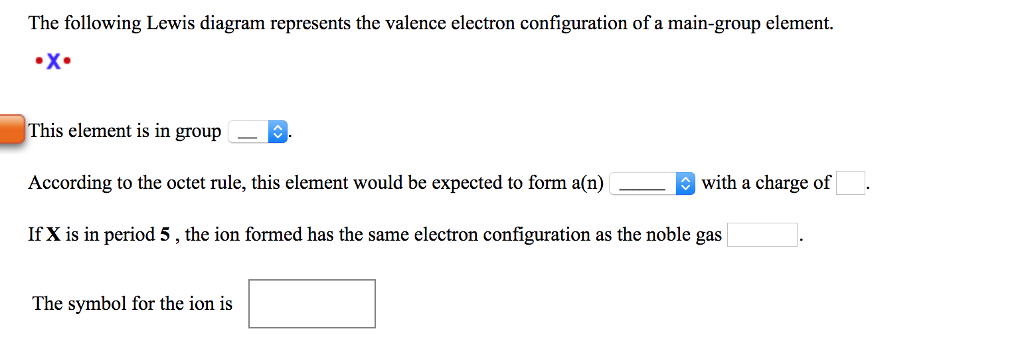
The following lewis diagram represents the valence electron configuration of a main-group element.. For representative (main group) elements, valence electrons are those electrons _____. which occupy the outermost s and p orbitals. How many valence electrons does the representative (main group) element with the electron configuration 1s^2 2s^2 2p^6 3s^2 3p^5 possess? The following Lewis diagram represents the valence electron configuration of a main-group element. X This element is in group According to the octet rule, this element would be expected to form an ion with a charge of If X is in period 5, the ion formed has the same electron configuration as the noble gas The symbol for the ion is Submit Answer ... Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group ... 26.11.2021 · When you are finished drawing your 2D structure, click on the Get Lewis Dot Structure button to see the result. Enter appropriate values in all cells except the one you wish to calculate. Powell, D. An online electron configuration calculator helps you to …
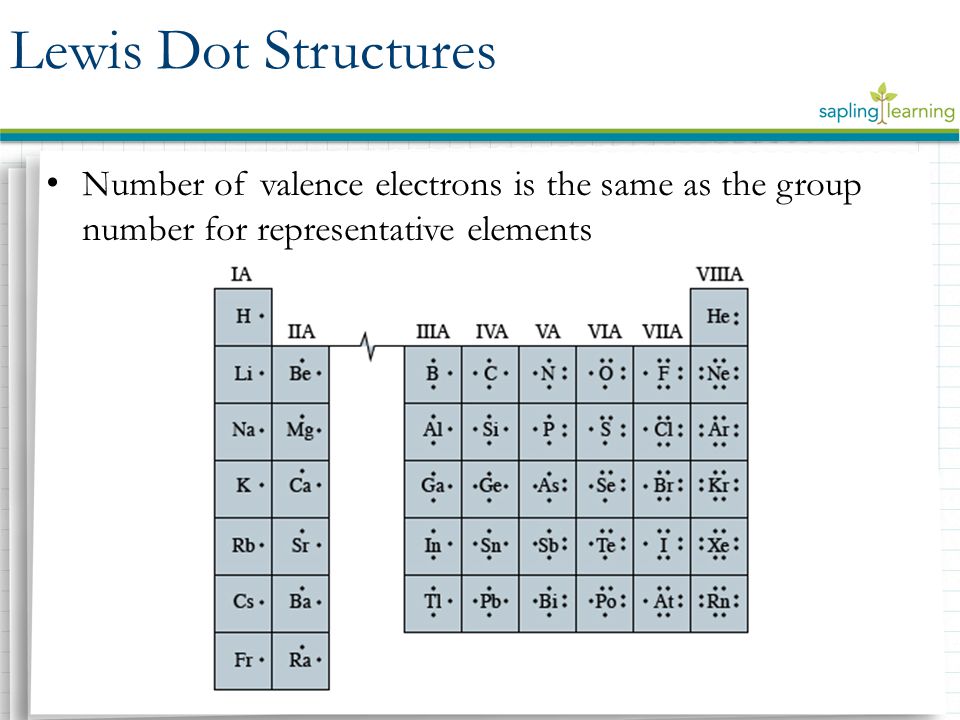
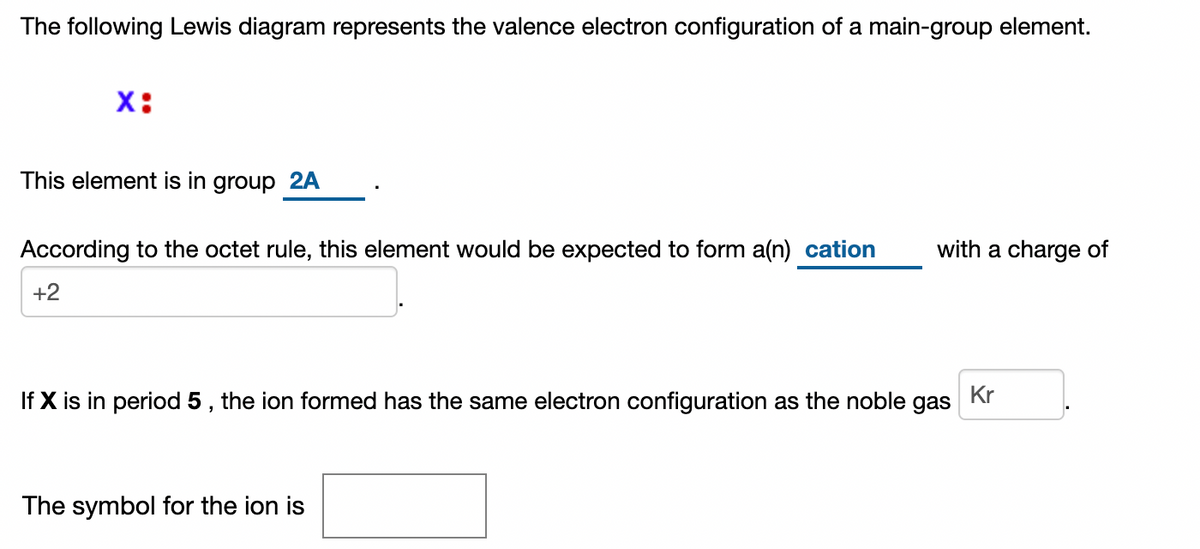
The Periodic Table was designed with this feature in mind. Each element has a number of valence electrons equal to its group number on the Periodic Table. Figure %: The periodicity of valence electrons This table illustrates a number of interesting, and complicating, features of electron configuration. The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group 2A According to the octet rule, this element would be expected to form a(n) with a charge of cation anion If X is in period 4, the ion formed has the same electron configuration as the noble gas The symbol for the ion is_____ Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element, If this element is in period 4, ... The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group 2A According to the octet rule, this element would be expected to form a(n) with a charge of cation anion If X is in period 4, the ion formed has the same electron configuration as the noble gas The symbol for the ion is
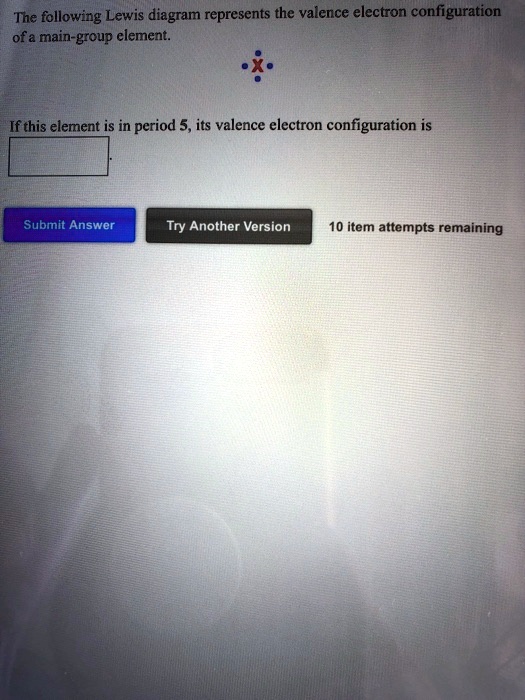
5.3: Lewis Diagrams. Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom. The kernel of the atom, i.e., the nucleus together with the inner electrons, is represented by the chemical symbol, and only the valence electrons are drawn as dots ... Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. If this element is in period 5, ... Note: number of dots = number of valence electrons = group number. A) The following Lewis diagram represents the valence electron configuration of a main-group element. The Lewis diagram shows 3 valence electrons, and represents a valence electron configuration of the type ns 2 np 1. Elements with 3 valence electrons are in group 3A. 1. Write the correct skeletal structure for the molecule 2. Calculate the total number of electrons for the Lewis structure by summing the valence electrons of each atom in the molecule (If you are writing a Lewis structure for a polyatomic ion, you must consider the charge of the ion when calculating the total number of electrons) 3.
The following Lewis diagram represents the valence electron configuration of a main-group element. If this element is in period 2, its valence electron configuration is The formal charge is the "charge" an element would have in a molecule or ion if all of the bonding clectrons were shared equally between atoms :0-5=Based on the Lewis ...
FREE Answer to The following Lewis diagram represents the valence electron configuration of a main-group element. X• The element...1 answer · Top answer: From the data this has 1 valence electrone 1 Valence electron has 'I' Group elementti Given 6th period element, and iie. 6th period, I Group element is ...
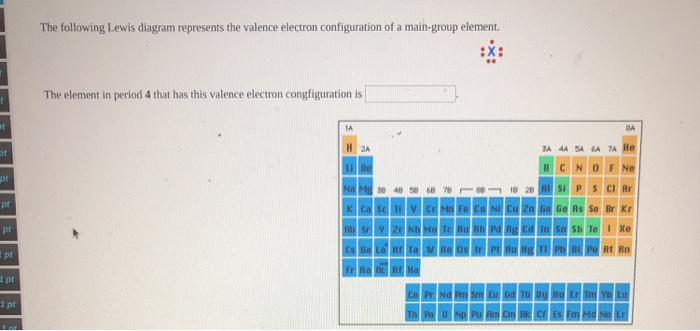
The following Lewis diagram represents the valence electron configuration of a main-group element. The element in period 2 that has this valence electron congfiguration is He Ne Na Mg 38 Ca Sc Cr Mn Fe Co Cu Zn Ga Ge As Kr Rb Nb Mo Tc Ru Rh Pd Ag Cd Ba La ]Hf Re Au Hg Ra Ac Rf Ha Sn Sb Xe Po At Rn Ce Nd PmSm Gd Tb Dy Ho Tm Yb Lu Np Pu Am Cm BkCf Es Fm Md No
cess important values if needed The following Lewis diagram represents the valence electron configuration of a main-group element. X• This element is in group According to the octet rule, this element would be expected to form an ion with a charge of If X is in period 4, the ion formed has the same electron configuration as the noble gas The symbol for the ion is
Start studying CHM2045C Chemistry I Final Exam. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
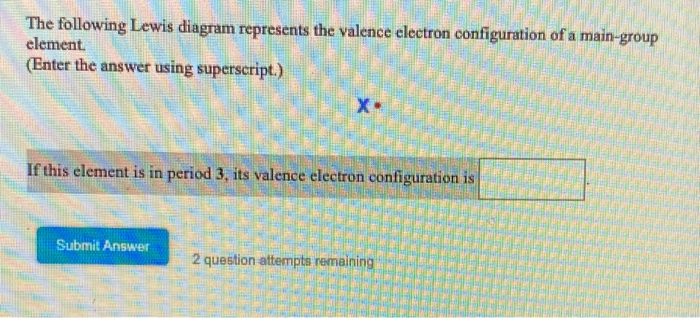
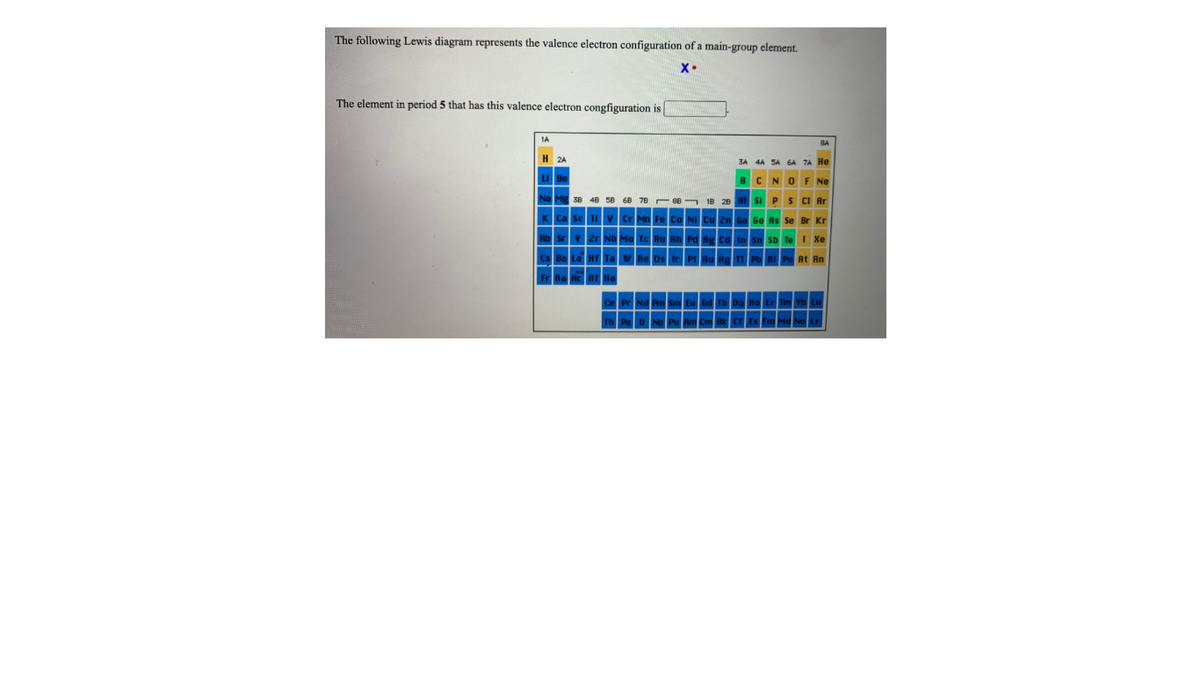
The following Lewis diagram represents the valence electron configuration of a main-group element. If this element is in period 3, its valence electron configuration is The following Lewis diagram represents the valence electron configuration of a main-group element. •X: The element in period 5 that has this valence electron congfiguration is
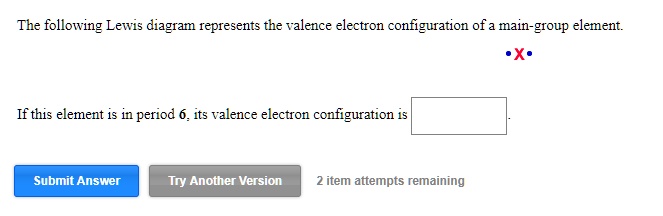
The Electron Configuration Video Lessons. Concept: Concept: Example: Problem: The following Lewis diagram represents the valence electron configuration of a main-group element. If this element is in period 6, its valence electron configuration is:
Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
The following Lewis diagram represents the valence electron configuration of a main-group element. The element in period 4 that has this valence electron congfiguration is at 1A BA ot H 2 BA 4A SA GA 7A He LiBe ot pe B C N O F Ne Na MENO 40 5 6 8 2 AISI PSC Ar K CSC CE MA FI CON CU 20 Ga Ge As Se Br Kr RESEN MOTRU A PO Rg Ca S Sb Te Cs Ba La Hf Ta W Re Os Ir
The following Lewis diagram represents the valence electron configuration of a main-group element.-B This element is in group According to the octet rule, this element would be expected to form a (n)with a charge of If X is in period 5, the ion formed has the same electron configuration as the noble gas The symbol for the ion is.
The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group According to the octet rule, this element would be expected to form a(n) with a charge of If X is in period 4 , the ion formed has the same electron configuration as the noble gas The symbol for the ion is Submit Answer Retry Entire Group 7 more group attempts remaining ...
Science. Chemistry. Chemistry questions and answers. The following Lewis diagram represents the valence electron configuration of a main-group element. .: The element in period 4 that has this valence electron congfiguration is 1A BA H 2A ЗА 4A SA SA 7A Не Li Be B C N O F Ne Na Mg 38 48 58 6B 7B 8B B2B Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe ...
Determine in which block of the periodic table are the elements having the filling valence electrons configuration. ... Identify each of the following as a representative element or transition element. Promethium (Pm) ... Determine the group, period, and block of an atom with the following electron configurations: (Ne)3s2. Group 2, Period 3 ...
A core-abbreviated electron configuration (right) replaces the core electrons with the noble gas symbol whose configuration matches the core electron configuration of the other element. Similarly, the abbreviated configuration of lithium can be represented as [He]2 s 1 , where [He] represents the configuration of the helium atom, which is identical to that of the filled inner shell of lithium.
37 the following lewis diagram represents the valence electron configuration of a main-group element. Written By Stephanie J. Cox. ... The valence electron s of an atom are shown in an electron dot diagram. In chemistry, a valence electron is an electron that is associated with an atom, ...
The following Lewis diagram represents the valence electron configuration of a main-group element. X• This element is in group According to the octet rule, this element would be expected to form a(n) v with a charge of If X is in period 3 , the ion formed has the same electron configuration as the noble gas The symbol for the ion is <>
Lewis structure: diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion. Lewis symbol: symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion. lone pair: two (a pair of) valence electrons that are not used to form a covalent bond.
The Lewis Electron-Dot Symbols of Elements. Gilbert N Lewis is widely known for his use of simple symbolic representations of elements that show valence electrons as dots. You've seen the Bohr's diagram for the first 18 elements. Sometimes it is more convenient to represent the elements by its Lewis electron dot symbol.
Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group 1A ...
The following Lewis diagram represents the valence electron configuration of a main-group element. X• This element is in group According to the octet rule, this element would be expected to form a(n) with a charge of If X is in period 3 , the ion formed has the same electron configuration as the noble gas CO The symbol for the ion is
Solution. The valence electron configuration for aluminum is 3 s 2 3 p 1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons: A l: ˙. The valence electron configuration for selenium is 4 s 2 4 p 4. In the highest-numbered shell, the n = 4 shell, there are six electrons.
12.11.2018 · A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds.Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound.. Carbon tetrachloride (CCl 4) is a covalently bonded compound composed of a central carbon surrounded by 4 chlorine atoms in a …
Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group ...
Inorganic Chemistry (Atkins, Shriver).PDF
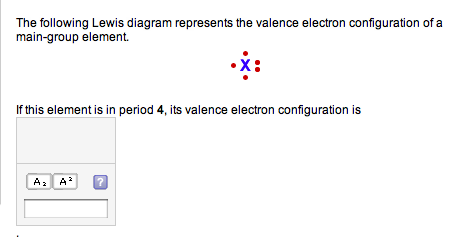
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
The following Lewis diagram represents the valence electron configuration of a main-group element. If this element is in period 6, its valence electron configuration is: Expert Q&A
(b) The following Lewis diagram represents the valence electron configuration of a main-group element. Identify the element in period 5 that has this valence electron configuration. SOLUTION: (a) Te Tellurium is a Group 6A element and has six valence electrons. (b) Rb The element has one valence electron, so it is a Group 1A element. Rubidium ...
the human questions from the topic. Electronic confirmation. And this question is asked that look at these elements in correctable and then draw withdrawal str…
A core-abbreviated electron configuration (right) replaces the core electrons with the noble gas symbol whose configuration matches the core electron configuration of the other element. Similarly, the abbreviated configuration of lithium can be represented as [He]2 s 1 , where [He] represents the configuration of the helium atom, which is identical to that of the filled inner shell of lithium.
ALL YOUR PAPER NEEDS COVERED 24/7. No matter what kind of academic paper you need, it is simple and affordable to place your order with Achiever Essays.
The following Lewis diagram represents the valence electron configuration of a main-group element EX. Show transcribed image text ...
A Lewis diagram shows all of the valence electrons in a species. Each dot in a Lewis diagram represents a single electron. A pair of dots on one atom symbol is called a lone pair. Lone pair electrons are non-bonding and "belong" to only one atom. Each line connecting two atom symbols represents a two-electron bond (a shared pair of electrons).
Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group 2A ...
The following Lewis diagram represents the valence electron configuration of a main-group element. •X If this element is in period 6, its valence electron configuration is Submit Answer Tutored Practice Problem 8.2.2 Draw Lewis structures. a) Draw a Lewis structure for PHz in which all atoms except hydrogen obey the octet rule. Do not draw double bonds to oxygen unless they are needed in ...
Academia.edu is a platform for academics to share research papers.
The following Lewis diagram represents the valence electron configuration of a main-group element. (Enter the aSWer using sup'eTScripl ) If this element [riod ils valence electron conliguration - Need help with homework? Try Numerade Free for 7 Days. Continue ...



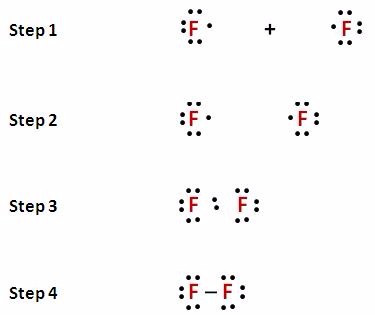










0 Response to "36 the following lewis diagram represents the valence electron configuration of a main-group element."
Post a Comment