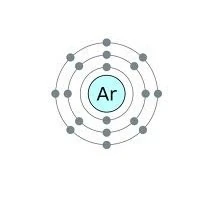
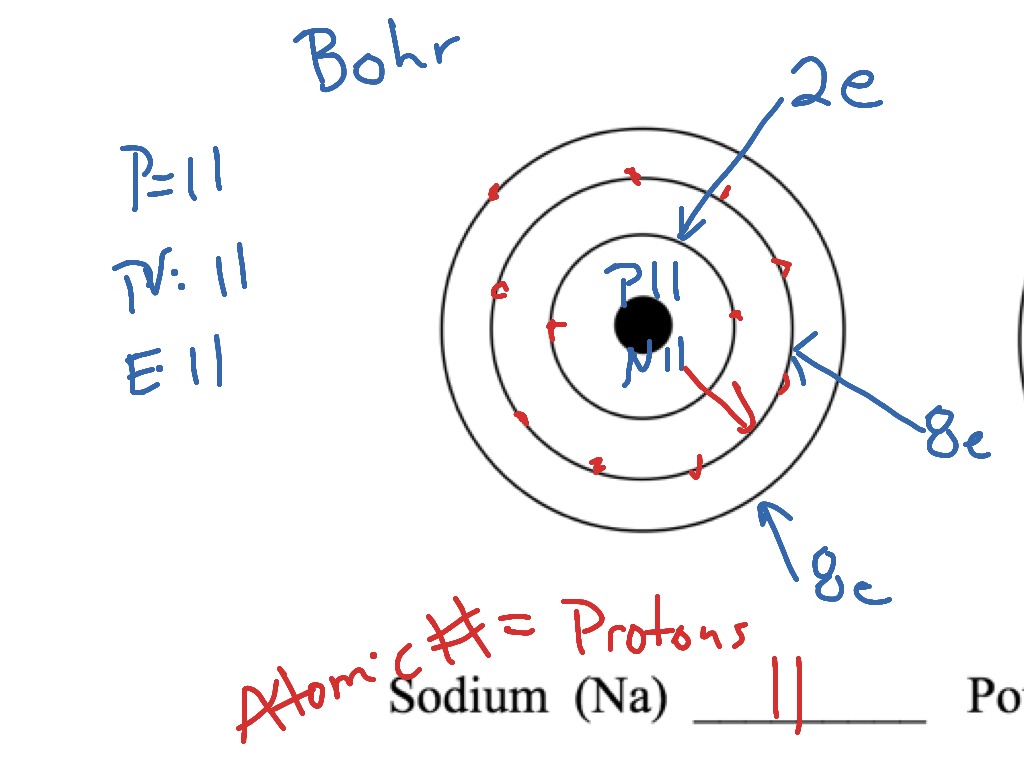
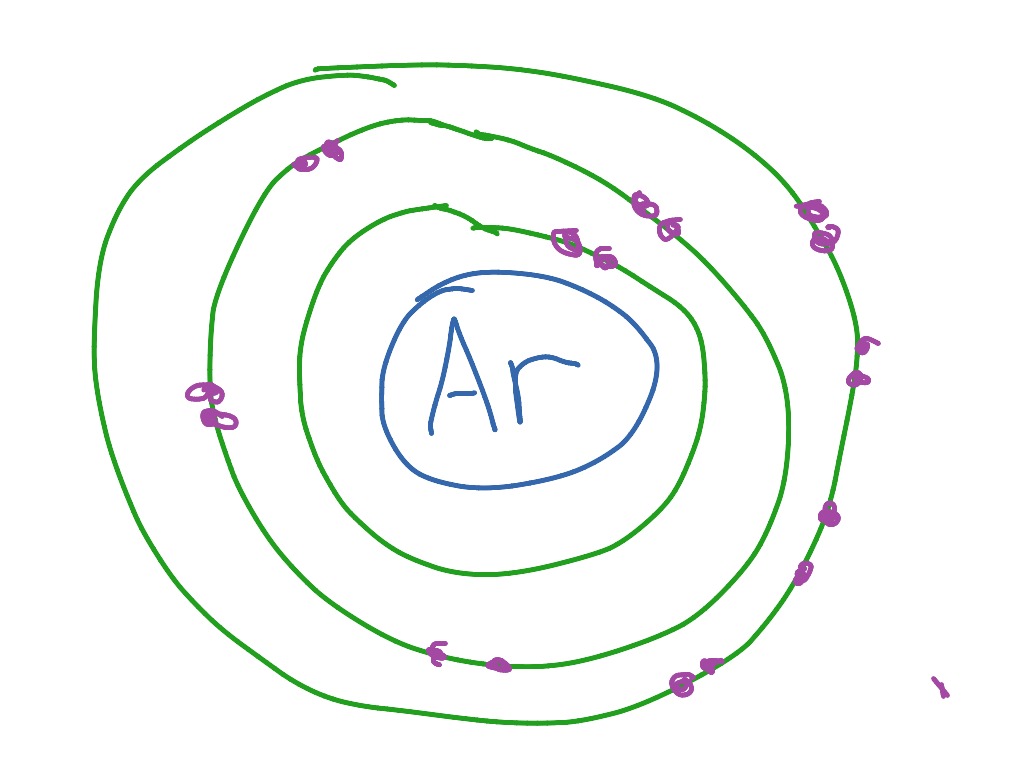
36 bohr diagram for argon
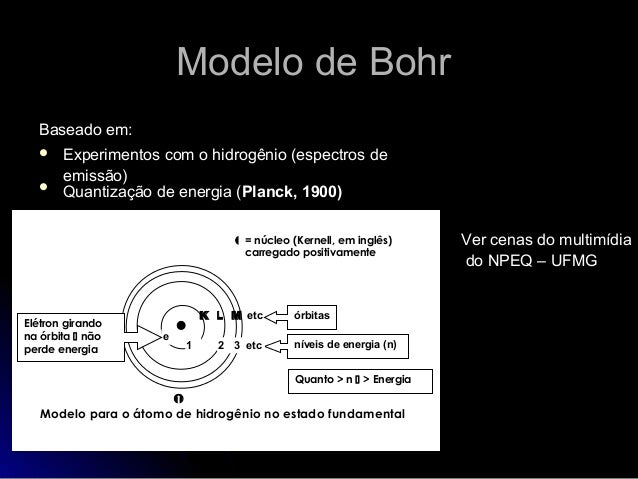
In atomic physics, the Bohr model or Rutherford-Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.After the solar system Joseph Larmor model (1897), the cubical model (1902 ... Bohr Diagrams in terms of A Bohr diagram is a diagram that shows how many each shell surrounding the nucleus. Named in honour of , a Danish physicist who developed several models for showing the arrangement of electrons in atoms. There are three main background questions to explore before we start drawing Bohr diagrams.
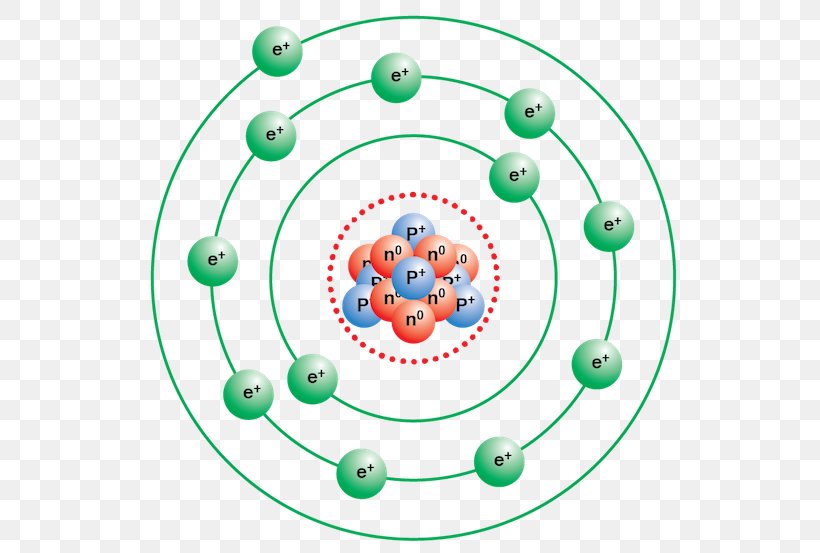
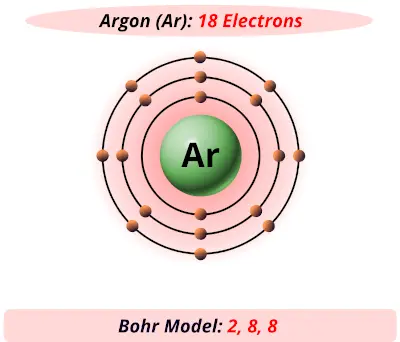
Argon has 2 electrons in its first shell, 8 in its second, 8 in its third.Check me out: http://www.chemistnate.com

Bohr diagram for argon
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons argon atom chlorine atom chlorine ion potassium atom potassium ion Atomic number Number of 10 protons 10 2. Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3. What do you notice about the arrangement of electrons in the Bohr model of a ... Here is a bohr model for Argon: We draw dots to represent the electrons. Notice how there are 2 electrons in the first shell, 8 electrons in the second, and 8 electrons in the third, making a total of 18 electrons. Thus, the atom is neutral. Click to read full answer. Accordingly, what type of bond does Argon form?
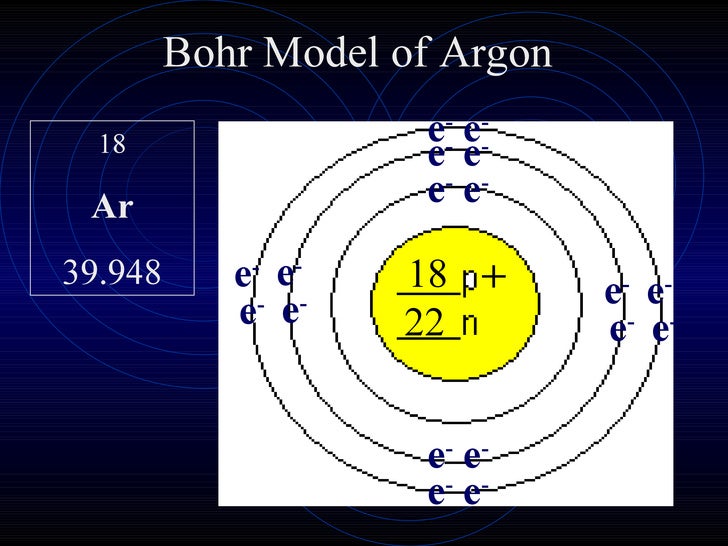
Bohr diagram for argon. Boron is neutral and its atomic number is 5, hence, the number of protons and electrons available for its Bohr diagram is also 5. What does argon atom look like? The argon atom has 18 electrons and 18 protons. Its outer shell is full with eight electrons. Under standard conditions argon is an odorless and colorless gas. Find step-by-step Biology solutions and your answer to the following textbook question: Draw Bohr-Rutherford diagram for the argon-40 atom.. Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He Bohr Model. A Bohr Model is a diagram of the number of electrons in each of the energy level (shell) around the nucleus of an atom. ... Argon. What element is this? It has 2 + 8 + 8 = 18 electrons, and therefore 18 protons. The element with atomic number of 18 is Argon. 18 p. 22 n.
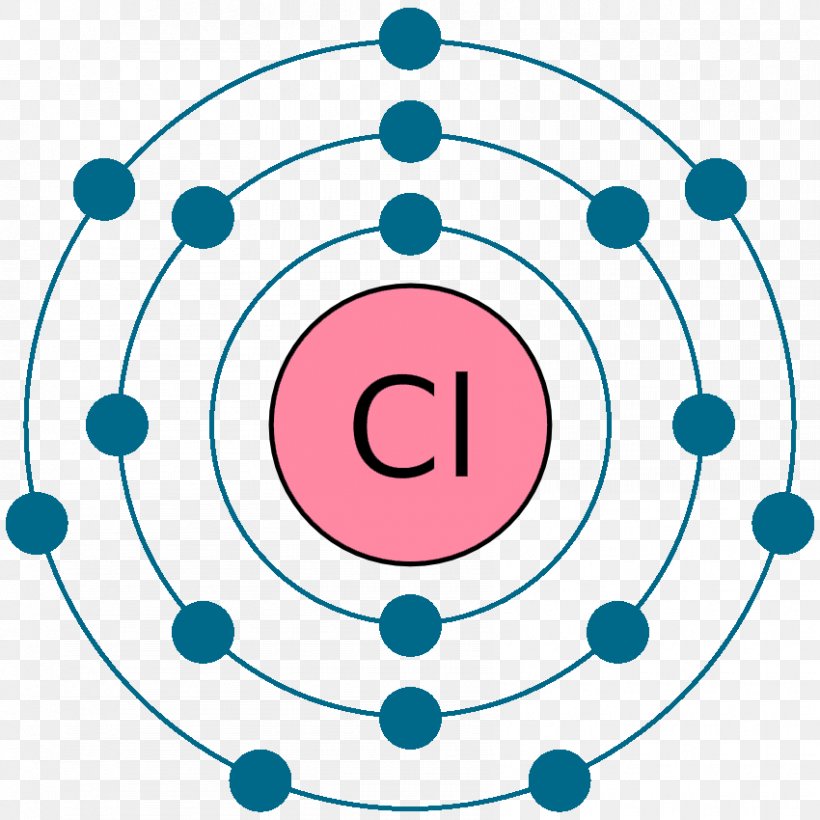
• A Bohr diagram is a diagram that shows how many _____ are in each shell surrounding the nucleus. • Named in honour of _____, a Danish physicist who ... argon a:om chlorine atom . potassium atom . 2.Use the table above to draw the Bohr model diagram for the following atoms and ions. Bohr Diagram For Argon. Here is a typical Bohr model, Draw a Bohr Model for an Argon atom. How many neutrons and protons does it have? How many electrons does. Last class, we determined that the Bohr Model is a planetary model in which the For example, there are 3 shells in the bohr diagram of Argon. Bohr diagrams indicate how many electrons fill each principal shell. Aug 14, 2018 · on Bohr Diagram For Argon. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer. Bohr Model Of Argon Atom Potassium Atom, Copper Atom, Atom Model Project, Bohr. Visit chemical elements, crystals, melting points, [Bohr Model of Copper]. Bromine has 18 electrons in its third shell because it is past Zinc on the periodic table. Then, you go back to the fourth shell and put 5 extra electrons in...
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Argon has 18 electrons. 1 . Unit 1 Chemistry - Naming . Name:_____ Unit 1 Evaluation Notes filled in Assignments completed Answers corrected argon. what is the bohr model of nitrogen the bohr model for nitrogen has a central nucleus with seven neutrons and seven protons a first energy ring with two electrons and a second energy ring with five electrons a more detailed version shows two electrons in the s sub shell of the second energy ring and three electrons in the p sub shell what … Bohr diagram for argon the bohr electron configuration is 2 8 with innermost shell having two electrons outermost has eight pictures of argon valence electrons this diagram of a thorium atom shows the electron shell chemical elements com argon ar. 4 The 2nd shell can hold up to 8 electrons.
Here is a bohr model for Argon: We draw dots to represent the electrons. Notice how there are 2 electrons in the first shell, 8 electrons in the second, and 8 electrons in the third, making a total of 18 electrons. Thus, the atom is neutral. Also Know, what are the protons neutrons and electrons of fluorine?
Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of Aluminum (Al) 2, 8, 3: 14: Bohr model of ...
On the 2nd circle, draw 8 electrons. And finally, on the last circle, draw another 8 electrons. That is the bohr-rutherford diagram of argon.
The Bohr Diagram The Bohr Diagram is what scientists use to explain and show an atom's subatomic particles. This technique was created by two scientists in 1913. They are: Niels Bohr and Ernest Rutherford. [14] This drawing is very simple and easy to do. The number of outer shells an atom has is the number of circles drawn. (Example on page 3).
Sep 24, 2018 · Chemical schematron.org - Argon. Apr 24, · A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels.File argon (Ar) Bohr schematron.org - Wikimedia CommonsHow to Do Bohr Diagrams | Sciencing
Bohr’s diagram of Argon has three electron shells (K, L, and M), the inner shell is K-shell and the outermost shell is M-shell. Hence, the electrons found in the M-shell of the Argon atom are its valence electrons because it is the outermost shell that also called the valence shell.
Bohr diagram: Protons,Electrons,and Neutrons: 18 electrons so 8 are on the valance and the element is stable. ... Argon is used for welding,cutting,and spraying metals. Boiling point 185.85 degrees C.Melting point 189.35 degrees C. Argon cost $2.80 or $4.80 argon comes from the Greek word "argos" meaning "lazy" or "inactive."
To draw the Bohr model of an atom, we should follow 4 or 5 basic steps. Find the number of protons, electrons, and neutrons of an atom. Draw the nucleus of an atom. Write the number of protons and neutrons at the center of the nucleus. Draw the first electron shell and put the electrons as a dot in it.
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. ... What is the Bohr model for Argon? Here is a bohr model for Argon: We draw dots to represent the electrons. Notice how there are 2 electrons in the first shell, 8 electrons in the second, and 8 electrons in the third, making a total of 18 ...
What does the bohr diagram for carbon look like? I assume you mean Bohr, as in Neils Bohr, the physicist and chemist famous for the Bohr model of the atom.Imagine a "C" inside of a square.
Draw a Bohr model for elements with atomic numbers 1-18 (Hydrogen thru Argon). Draw each diagram neatly, clearly labeling the correct number of protons, electrons, and neutrons for each diagram. Use the average atomic mass from the periodic table for the mass number (round to the nearest whole number).
Here is a bohr model for Argon: We draw dots to represent the electrons. Notice how there are 2 electrons in the first shell, 8 electrons in the second, and 8 electrons in the third, making a total of 18 electrons. Thus, the atom is neutral. Click to read full answer. Accordingly, what type of bond does Argon form?
argon atom chlorine atom chlorine ion potassium atom potassium ion Atomic number Number of 10 protons 10 2. Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3. What do you notice about the arrangement of electrons in the Bohr model of a ...
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons




:max_bytes(150000):strip_icc()/hydrogenatom-58b6029c3df78cdcd83d98bb.jpg)



_Bohr_model.png)




0 Response to "36 bohr diagram for argon"
Post a Comment