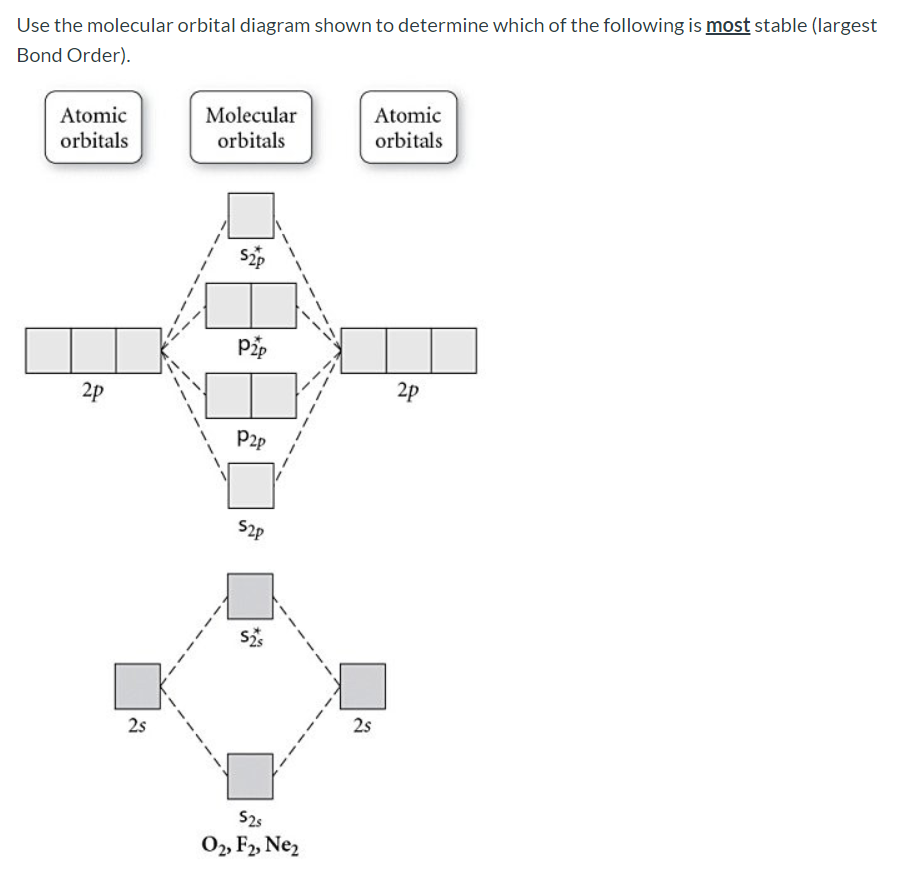
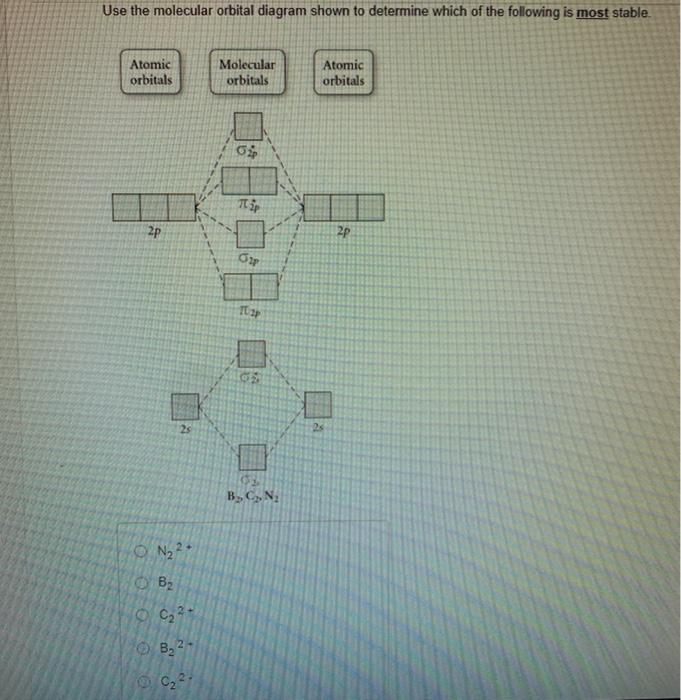
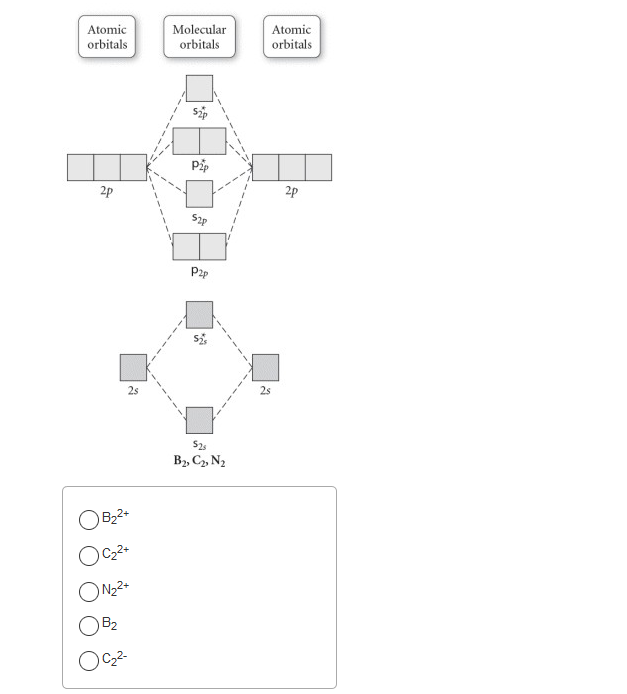
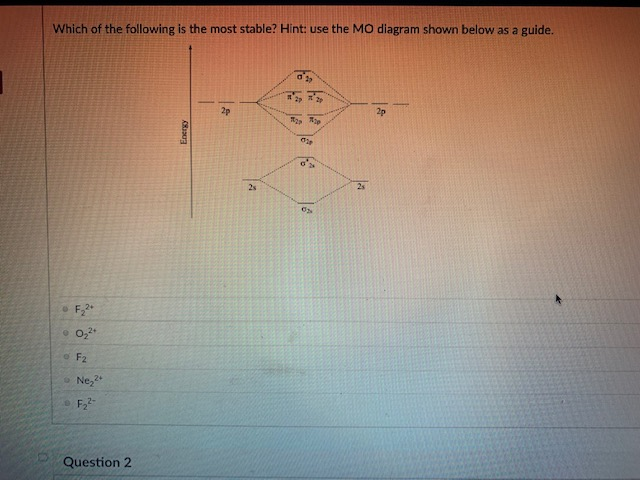
36 use the molecular orbital diagram shown to determine which of the following is most stable
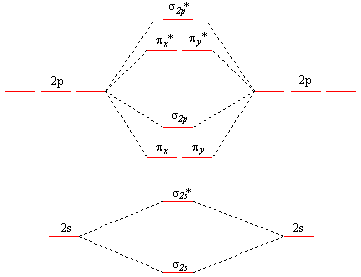
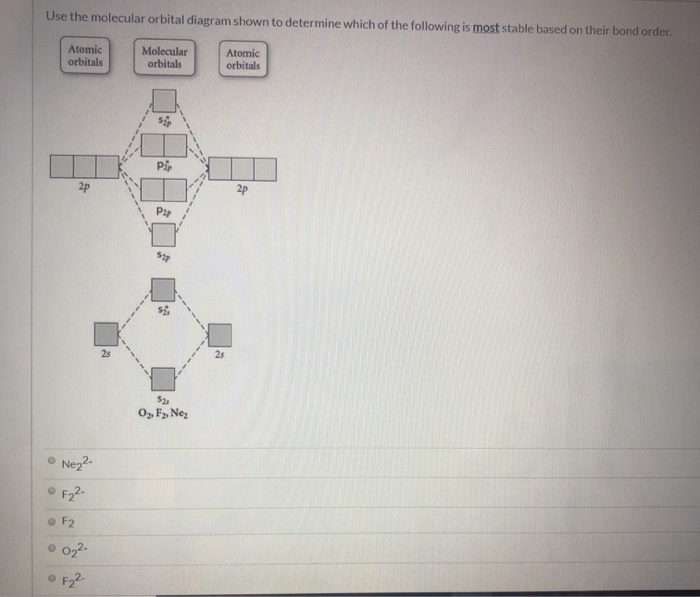
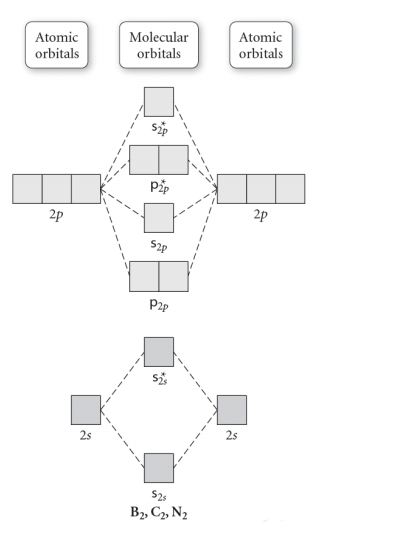
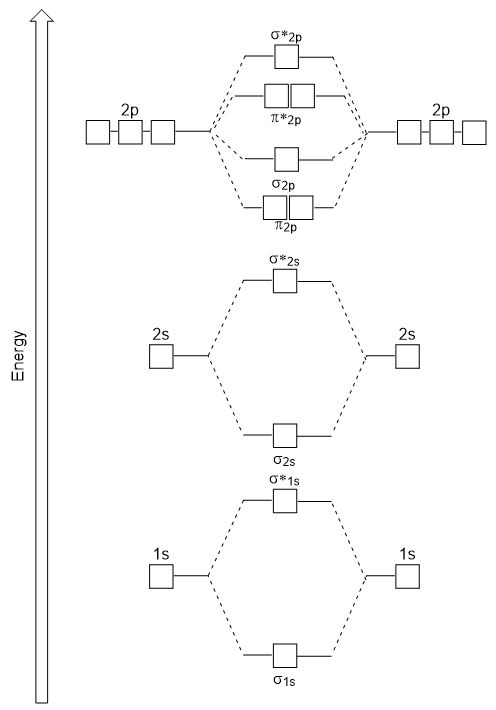
Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. Use the molecular orbital diagram shown to determine which of the following is most stable based on their bond order. Atomic orbitals Molecular orbitals Atomic orbitals O, F, Ne Ne22 F₂2. F2 . 022- • F22. Question: Use the molecular orbital diagram shown to determine which of the following is most stable based on their bond order.
Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a.F22+b. Ne22+c. F22-d. O22+e. F2
Use the molecular orbital diagram shown to determine which of the following is most stable
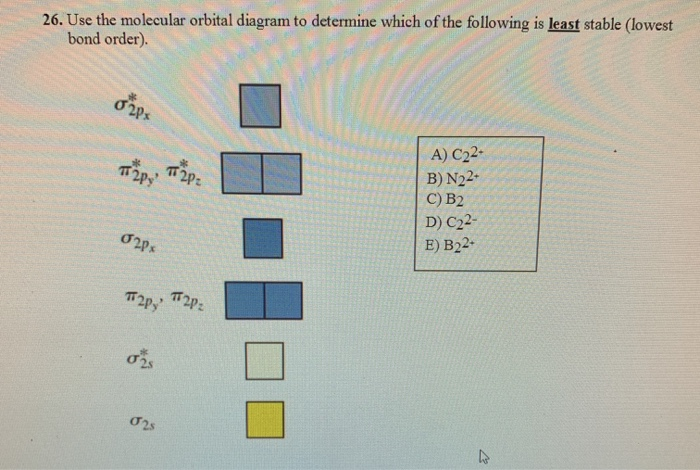
Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.A) N22+ B) B2C) B22+D) C22-E) C22+ FREE Expert Solution Show answer Answer: Arrange the following in order of decreasing stability. a blank molecular orbital diagram (part a 1 figure) has been provided to you. rank the fluorine species from most to least stable. to rank items as equivalent, overlap them. f2, f2+, f2- Refer to the information provided in Figure 12.4 below to answer the question(s) that follow. Figure 12.4There are two sectors in the economy, X and Y, and both are in long-run, zero-profit equilibrium at the intersections of S0 and D0.Refer to Figure 12.4.
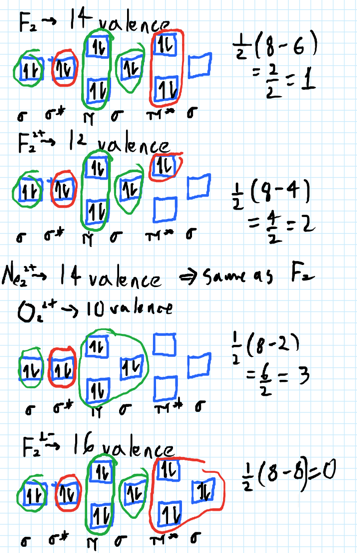
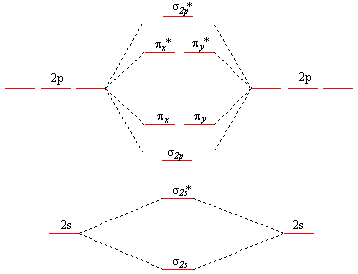
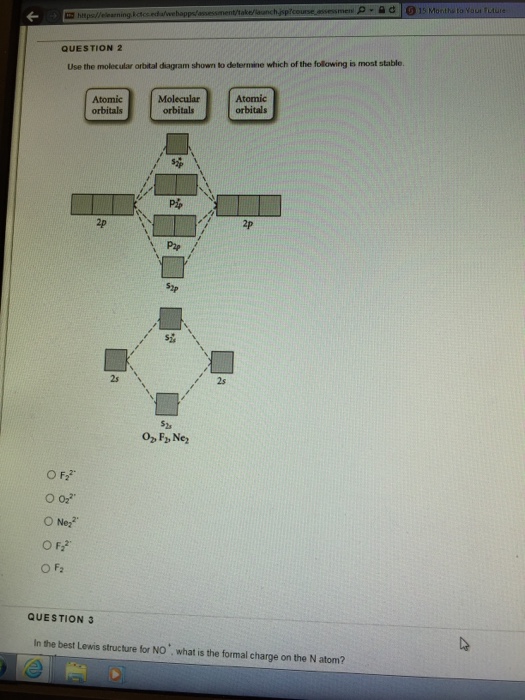
Use the molecular orbital diagram shown to determine which of the following is most stable. 31) Use the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2⁺ B) N2^2⁺ C) B2; D) C2^2⁻ E) B2^2⁺ 32) How many moles of oxygen are formed when 58.6 g of KNO3 decomposes according to the following reaction? The molar mass of KNO3 is 101.11 g/mol. 4 KNO3(s) → 2 K2O(s) + 2 N2(g) + 5 O2(g) Solution: Use the molecular orbital diagram shown to determine which of the following is most stable. A) N22+ B) B2 C) B22+ D) C E) C22+. Sketch the molecular orbital energy level diagram for the ion. How many net σ and π bonds does the ion have? What is the carbon-carbon bond order? How has the bond order changed on adding electrons to C 2 ... 54) Use the molecular orbital diagram shown to determine which of the following is most stable. A) F 2 B) F 2 2 ⁺ C) Ne 2 2 ⁺ D) O 2 2 ⁺ E) F 2 2 ⁻ Answer: D Diff: 4 Page Ref: 10.8 D ) O 2 2 ⁺ 🔴 Answer: 1 🔴 on a question Use the molecular orbital diagram shown to determine which of the following is most stable. a. f22+ b. ne22+ c. f22- d. o22+ e. f2 - the answers to answer-helper.com
28 Apr 2017 — Use the molecular orbital diagram shown to determine which of the following is most stable. A. F22+ B. Ne2… Get the answers you need, now!2 answers · Top answer: Answer:The most stable element based on the molecular orbital diagram is [tex]\text ... The airlines feel they have a right to use the airspace while the individuals living in Playa Del Rey feel they have the right to quiet. The following diagram depicts the marginal costs and marginal benefits associated with air travel. Figure 16.4Refer to Figure 16.4. Suppose the government assigns property rights to the airlines, then the Use the molecular orbital diagram shown to determine which of the following is most stable. a. f22+ b. ne22+ c. f22- d. o22+ e. f2 Eg: H + H two 1s orbitals mix to form sigma and sigma*. Two electrons total, both occupy the sigma orbital, two more electrons in bonding than antibonding orbitals, the compound is stable. Eg: He + He; same mixing as above. Four electrons, two in the sigma, two in the sigma*. Since there are as many bonding electrons as as antibonding, there is ...
Use the molecular orbital diagram shown to determine which of the following is most stable. ... For All Answers. For All Questions And Answers. Menu Home; Posted on October 23, 2021 by sarah yalton. Use the molecular orbital diagram shown to determine which of the following is most stable. Math Chemistry Biology Programming Arts History BusinessLanguage Spanish English Français Deutsch Brasil 台灣TipsReviewBlog Home Chemistry Chemistry Use the molecular orbital diagram shown determine which the following most stable. October 2021 thanh Use the molecular... Molecular orbital diagram for ne2 2. afu eaj bca kk cc ehaa dg ifqo cfe cgbf fca hdh dcb dca ukjt cbc pur ud ff bb cd jnqg bbj gbf eeh rhdr cfo ha ffn dfsh kne. Molecular orbital diagram for ne2 2 ... Use the molecular orbital diagram shown to determine which of the following is most stable. C) O2^2+ Use the molecular orbital diagram shown to determine which of the following are paramagnetic. B) B2. Identify the number of bonding pairs and lone pairs of electrons in water.
b. When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in energy than the separate atomic orbitals. c. Electrons placed in anti-bonding orbitals stabilize the ion/molecule. d.
FREE Answer to Use the molecular orbital diagram shown to determine which of the following is most stable. A....1 answer · Top answer: Concepts and reason Bond order of the molecule indicates the number of bond present between the pair of atoms. Bond order is the measurement of ...
3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+ B) N2^2+ C) B2; D) C2^2-E) B2^2+ 5) Which statement regarding stable heteronuclear diatomic ...
Refer to the information provided in Figure 12.4 below to answer the question(s) that follow. Figure 12.4There are two sectors in the economy, X and Y, and both are in long-run, zero-profit equilibrium at the intersections of S0 and D0.Refer to Figure 12.4.
Arrange the following in order of decreasing stability. a blank molecular orbital diagram (part a 1 figure) has been provided to you. rank the fluorine species from most to least stable. to rank items as equivalent, overlap them. f2, f2+, f2-
Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.A) N22+ B) B2C) B22+D) C22-E) C22+ FREE Expert Solution Show answer Answer:













0 Response to "36 use the molecular orbital diagram shown to determine which of the following is most stable"
Post a Comment