37 pb mg phase diagram
(Equi Diagram; Experimental; Indicates presence of a phase diagram.) Article Google Scholar 08Ste: N. J. Stepanow, “The Electrical Conductivity of Mg−Pb Alloys”,Z. Anorg. Chem., 60, 209–229 (1908) in German. (Equi Diagram; Experimental; Indicates presence of a phase diagram.) A ChemE freshman here, needing help. Would like to plot on screen first so my worksheet would be neat. Thanks!!
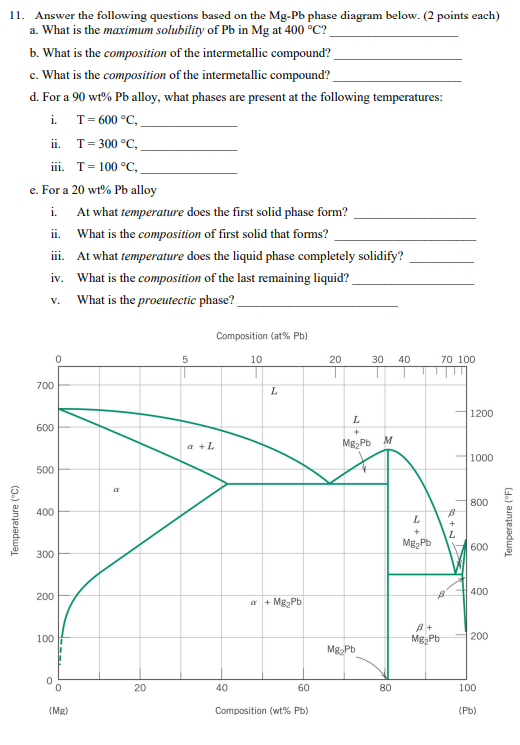
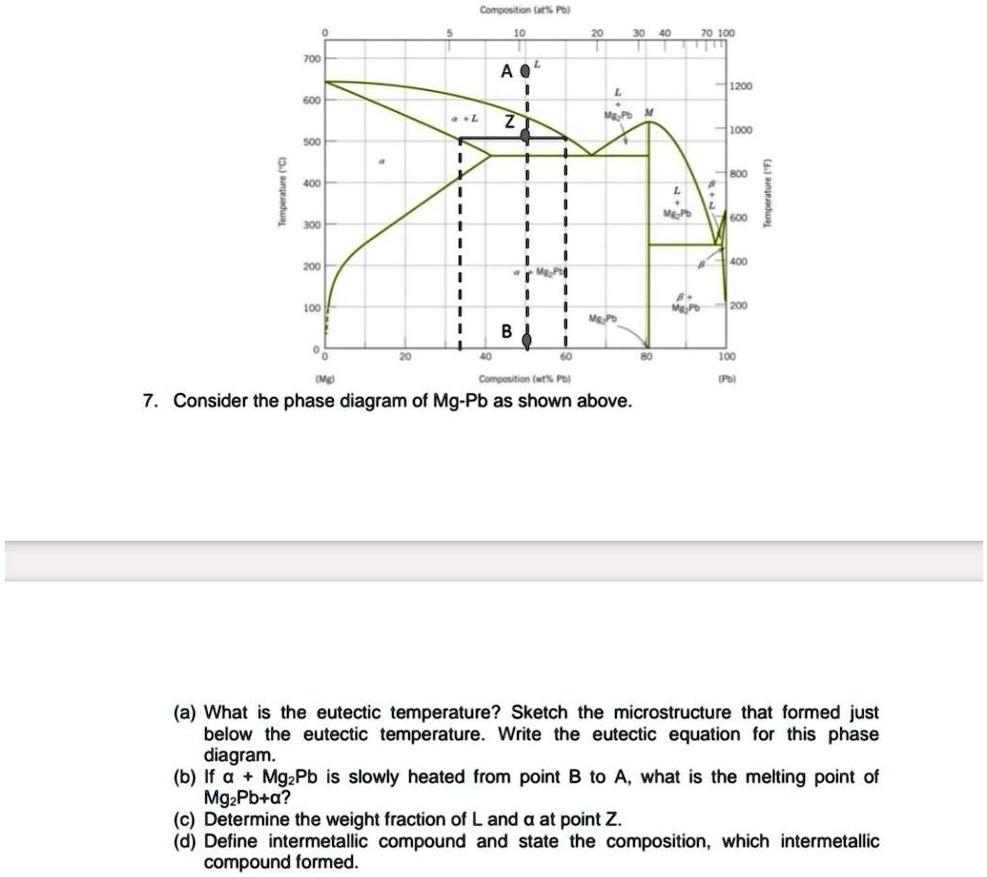
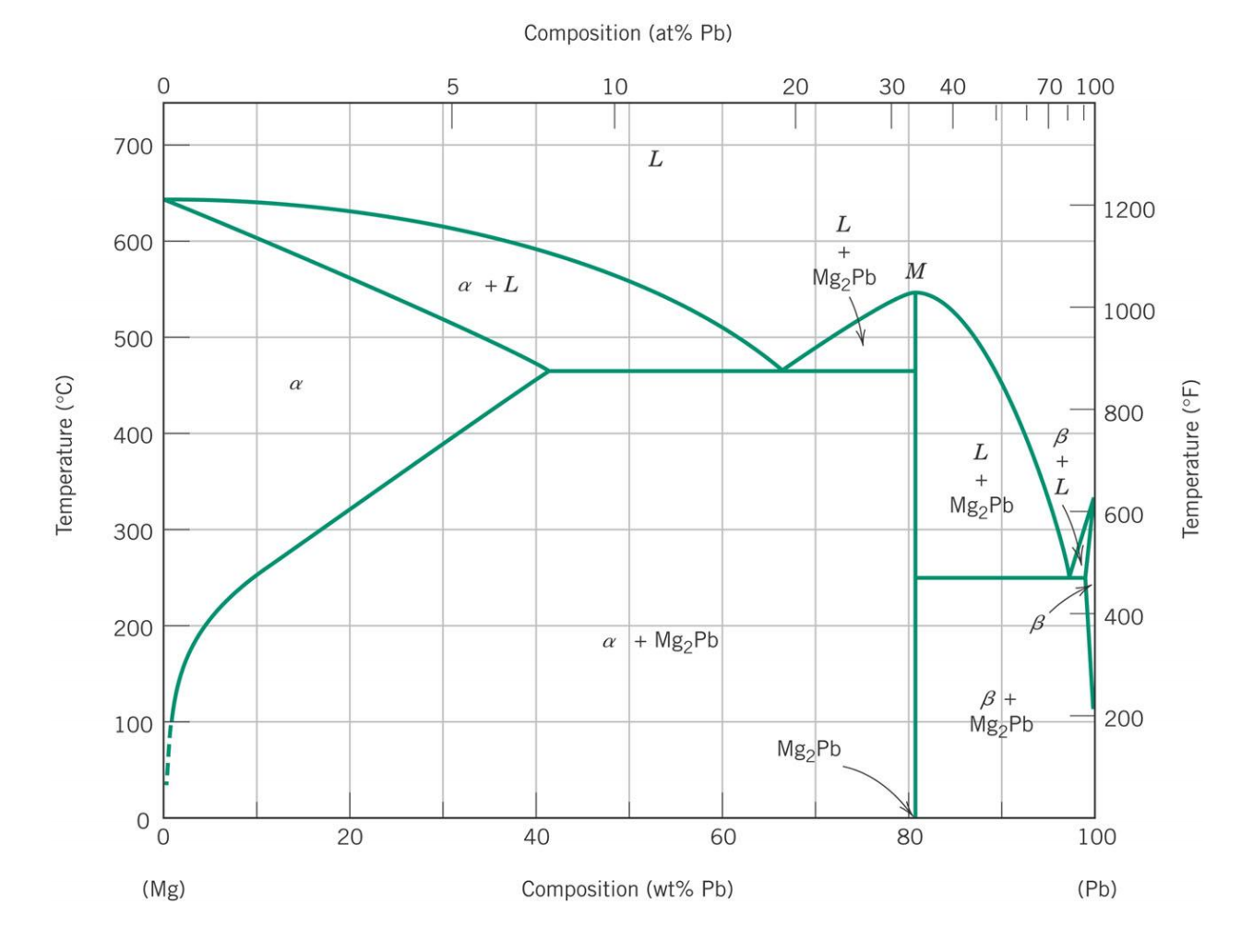
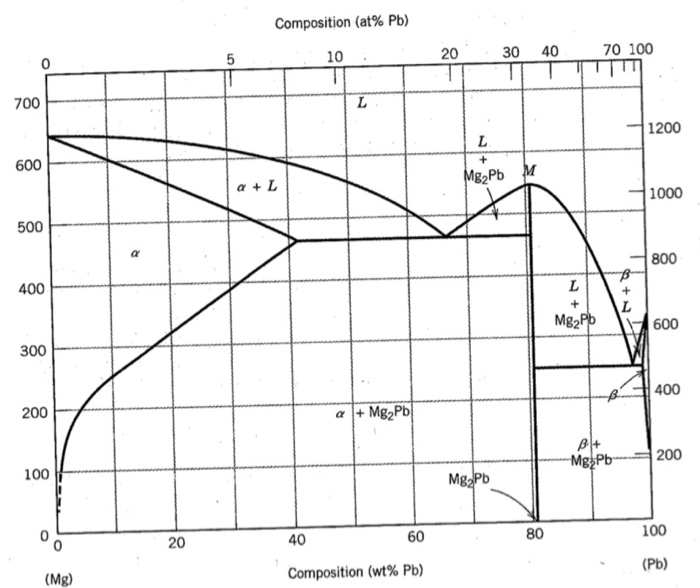
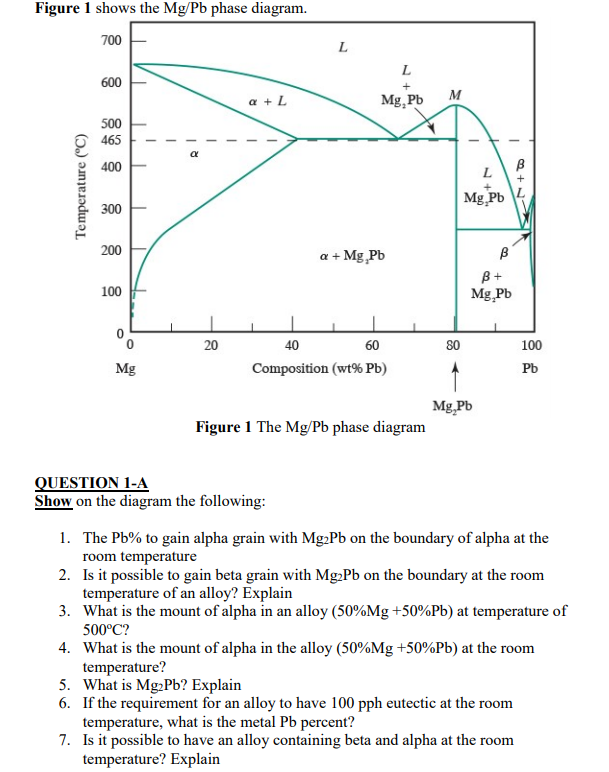
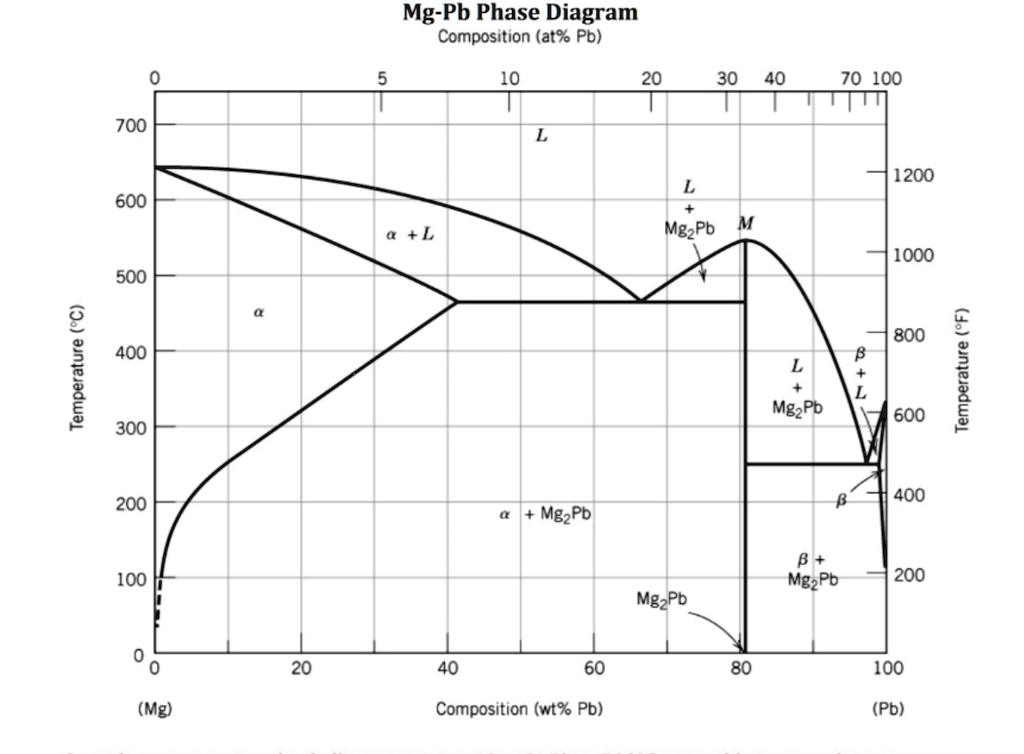
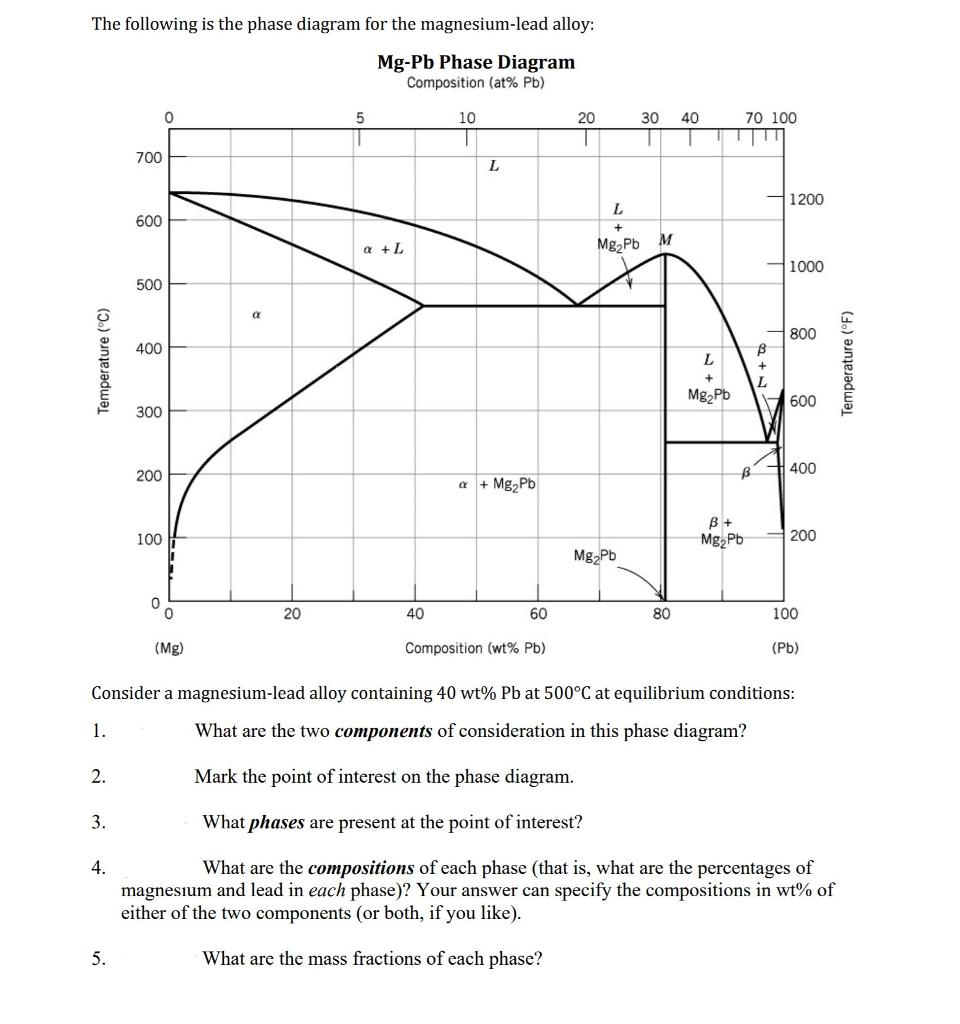
Shown below is the Mg-Pb phase diagram (Figure 9.20) and a vertical line constructed at a composition of 50 wt% Pb-50 wt% Mg. (a) Upon cooling from 700°C, the first solid phase forms at the temperature at which a vertical line at this composition intersects the L-(α + L) phase boundary--i.e., about 560°C;

Pb mg phase diagram
Abstract. The liquidus curve for the Mg-Pb system was redetermined, and its slope was used to assess phase transformations in the β' + Pb-rich liquid phase field. A peritectic reaction occurred at 538.7 °C when Mg 2 Pb decomposed into a Mg-rich liquid and β' (a Mg-Pb compound containing 35.15 at. pct Pb, or 0.3515 N Pb, the atomic fraction of Pb) which melted congruently at 548.5 °C, confirming the work of Eldridge et al. [1] When β' was cooled, a series of reactions occurred with the ... This video explains the Pb-Sn phase diagramFor further reading: https://www.physicsforums.com/threads/sn-pb-phase-diagram.281790/ Download scientific diagram | The phase diagram of the binary system, Pb–Mg. from publication: Eutectic Na–Tl and Pb–Mg alloys as liquid-metal coolants for fast nuclear reactors | The liquid ...
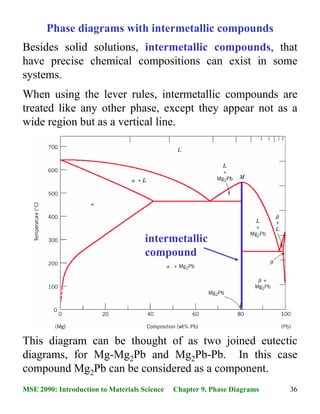
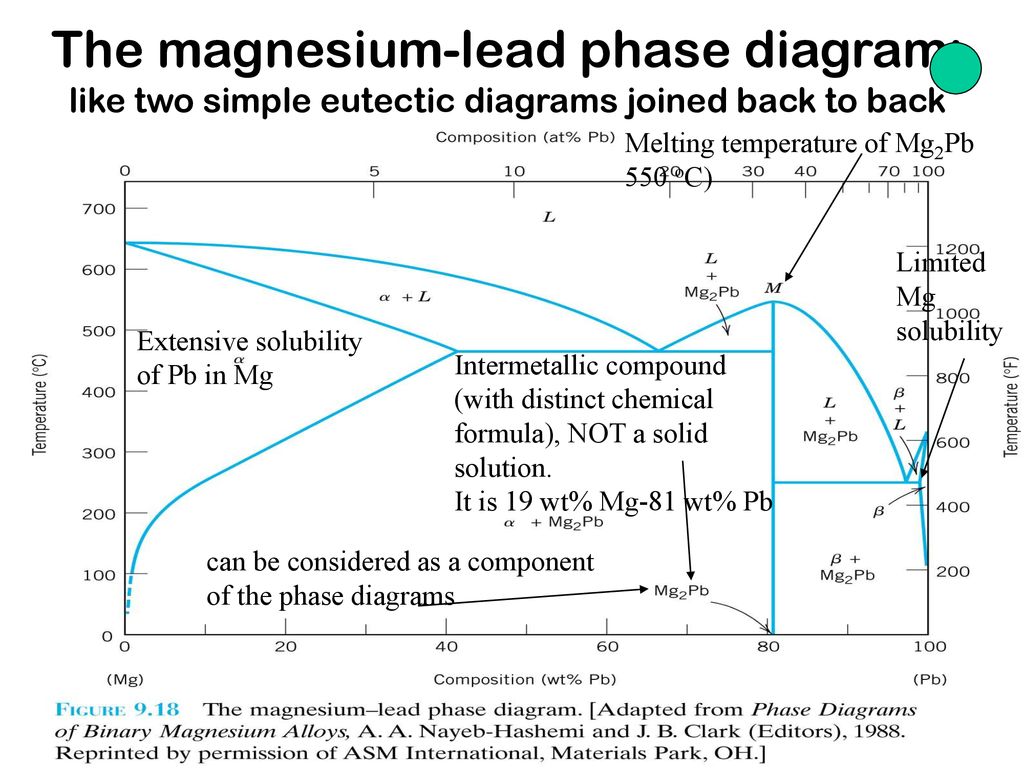
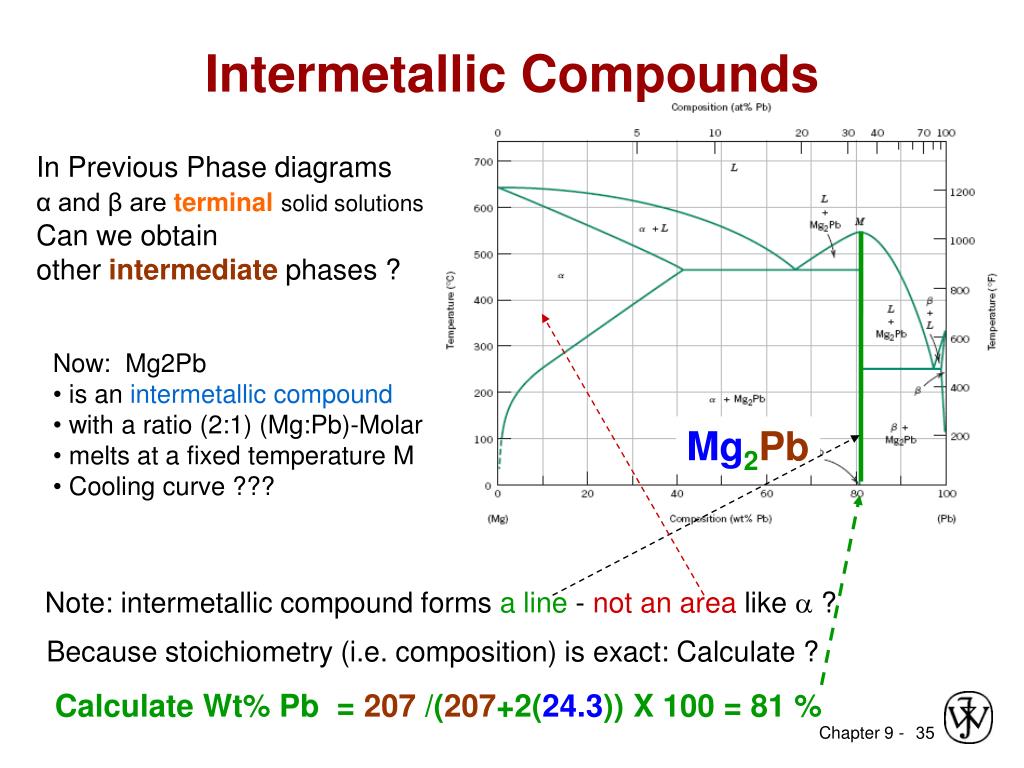
Pb mg phase diagram. Alloy Phase Diagrams 8 (1987) 326-334 Calculated Invariant Equilibria. Reaction Phase Mass % Ag Mass % Pb; L -> (Ag) + (Pb) 303.7 o C: Liquid: 2.27: 97.73 (Ag) 98.57: 1.43 (Pb) 0.10: 99.90: Phases, Crystal Structures and Model Descriptions. Phase Struktur-bericht Symbol Common Names Prototype Mg-Pb phase diagram and phase transformations in the intermetallic compounds Mg. 2. Pb and β. Siviour, N. G. ; Ng, K. Abstract. The liquidus curve for the Mg-Pb system was redetermined, and its slope was used to assess phase transformations in the β' + Pb-rich liquid phase field. A peritectic reaction occurred at 538.7 °C when Mg 2 Pb decomposed into a Mg-rich liquid and β' (a Mg-Pb compound containing 35.15 at. pct Pb, or 0.3515 N Pb, the atomic fraction of Pb) which melted congruently ... 16 The magnesium-lead phase diagram: like two simple eutectic diagrams joined back to back Intermetallic compound (with distinct chemical formula), NOT a solid solution. It is 19 wt% Mg-81 wt% Pb Melting temperature of Mg2Pb 550 oC) Extensive solubility of Pb in Mg Limited Mg solubility can be considered as a component of the phase diagrams 15 ... BINS - SGTE Free Binary Alloy Phase Diagrams (108) Click on a system to display the phase diagram.
It showed all their developmental products That portion of the Mg-Pb phase diagram (Figure 9.20) that pertains to this problem is shown below; the point labeled "F" represents the 85 wt% Pb-15 wt% Mg composition at 400°C. As may be noted, point F lies within the L + Mg 2Pb phase field. A tie line has been constructed at 400°C; it The Mg-Ca phase diagram and thermodynamic properties in Figures 8 and 9 are calculated based on their reported parameters as they showed better agreement with the available experimental data. The enthalpy and entropy of formation of obtained from different sources are summarized in Table 12. Solution The copper-gold phase diagram is constructed below. 9 Cite the phases that are present and the phase compositions for the following alloys: (a) 15 wt% Sn-85 wt% Pb at 100°C (212°F) (b) 25 wt% Pb-75 wt% Mg at 425°C (800°F) (c) 85 wt% Ag-15 wt% Cu at 800°C (1470°F)
https://imgur.com/a/x8XS0si What's the striped line? And what's up with the L (liquid) and glass? Something can't be glass and liquid simultaneously. Phase Struktur-bericht Symbol Common Names Prototype Spacegroup Model * Liquid: n/a: L: n/a: n/a (Bi,Pb) 1 : Fcc: A1 (Pb) Cu: Fm-3m (Bi,Pb) 1 (Va) 1: Hcp: A3 (epsilon Pb) Mg: P6 3 /mmc (Bi,Pb) 1 (Va) 0.5: Rho: A7 (Bi) alpha As: R-3m (Bi) 1 * (d) From Problem 9.8d, just the α phase is present for a 30 wt% Pb-70 wt% Mg alloy at 425 °C, as may be noted in the Mg-Pb phase diagram shown below (at point D)—i.e., W α = 1.0 Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for testing or instructional purposes only to The liquidus curve for the Mg-Pb system was redetermined, and its slope was used to assess phase transformations in theβ' + Pb-rich liquid phase field. A peritectic reaction occurred at 538.7 °C when Mg2Pb decomposed into a Mg-rich liquid andβ' (a Mg-Pb compound containing 35.15 at. pct Pb, or 0.3515 NPb, the atomic fraction of Pb) which melted congruently at 548.5 °C, confirming the work ...
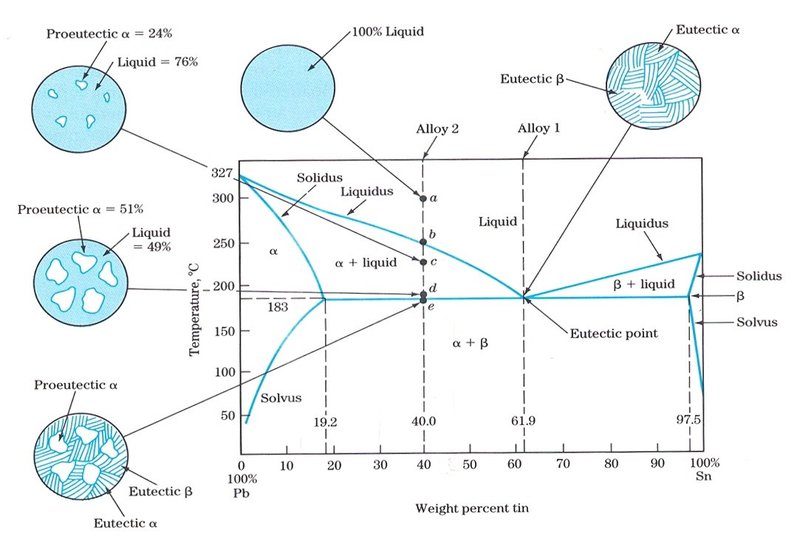
Solder material is taken at 62% Sn because it has the lowest metling point on the phase diagram. But even at 18% Sn the temperature is 183 celcius. Why not consider that. I know I'm wrong somewhere, can anyone point it out ?
I’d like to wire one of my guitars with 2 humbuckers with a volume and tone for each, a 3 way pickup selector switch, a 2 way switch to go out of phase, and a 2 way switch to split the coils. Like the wiring on the frank zappa Roxy SG.
SGTE - SGTE 2017 Alloy Phase Diagrams (1176) Click on a system to display the phase diagram.
Shown below is the Mg-Pb phase diagram (Figure 9.20) and a vertical line constructed at a composition of 50 wt% Pb-50 wt% Mg. (a) Upon cooling from 700°C, the first solid phase forms at the temperature at which a vertical line at this composition intersects the L-(a + L) phase boundary --i.e., about 560°C;
Solution Shown below is the Mg-Pb phase diagram (Figure 9.20) and a vertical line constructed at a composition of 50 wt% Pb-50 wt% Mg. (a) Upon cooling from 700°C (973 K), the first solid phase forms at the temperature at which a vertical line at this composition intersects the L − (α + L) phase boundary—i.e., about 560°C (833 K). ...
[Phase diagram](http://i.imgur.com/dm26qae.png) One question is: Researchers claimed to have found a Pb-Sn-alloy with a weight% of 90 % Pb. Can this be true, according to the following phase diagram? Explain why. If I look at this phase diagram at 90% Pb I can see, that there are four different regions depending on the temperature. Starting from the top: Liquid (Melt), Liquid + Pb(s), Pb(s) and Pb(s) + Sn(s). But how can I now if the Pb(s) + Sn(s) region is a mixed crystal and when it is a m...
We are asked to determine the approximate temperature from which a Pb-Mg alloy was quenched, given the mass fractions of α and Mg 2 Pb phases. We need to refer to the appropriate binary phase diagram, Fig. 9.18, p. 269, copied below: We can write a lever-rule expression for the mass fraction of the α phase as Wα = 0.65 = CMg 2 Pb - Co CMg 2 ...
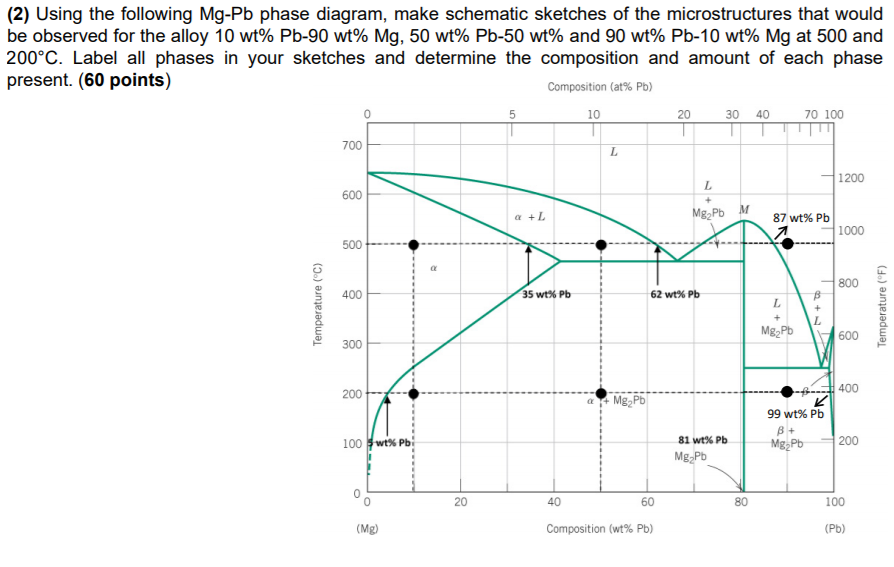
The magnesium-lead (Mg-Pb) phase diagram is shown in the figure below. Consider three Mg-Pb alloys of the following compositions: alloy #1: C1 = 20 wt.% Pb, alloy #2: C2 = 50 wt.% Pb, alloy #3: C3 = 90 wt.% Pb. Each of the alloys is slowly cooled down from 700. Question: The magnesium-lead (Mg-Pb) phase diagram is shown in the figure below.
This video is the first part in a series about phase diagrams. This video used the eutectic phase diagram to define terminology and phase diagram calculation...
Phase diagram and dielectric properties of Pb(In 1=2Nb 1=2)O 3-Pb(Mg 1=3Nb 2=3)O 3-PbTiO 3 ceramics Dabin Lin *,†, Haohua Chen , Zhenrong Li*,‡ and Zhuo Xu *Electronic Materials Research Laboratory Key Laboratory of Education Ministry/International Center for Dielectric Research Xi'an Jiao Tong University, Xi'an 710049, P. R. China
Phase diagram questions. 14:440:407 ch9 Question 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. What will be the composition of the ...
Report the maximum solubility of magnesium in lead and its temperature; c. Identify the point on the phase diagram associated with the temperature and ...1 answer · 0 votes: at 470C 41 wt % pb in mg %mg in pb at 250 c (6 I wt % atl A) Tec - 1 1 50wt% Pb @ phases :. pro eutectic liquid. 34 wt% pb 62 wt % pb L L 50 62 ...
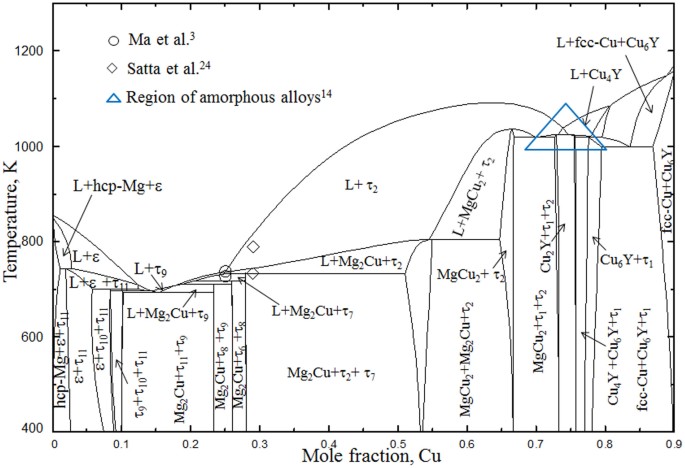
The available literature data of the Mg-Bi and Mg-Pb systems, including phase diagram and thermochemical properties data, were critically reviewed in the present work and summarized in Table 1. J. Min. Metall. Sect. B-Metall. 50 (2) B (2014) 115 - 126
A phase diagram shows what phases are present and where the process boundaries are within the composition space. Equilibrium phase diagrams represents relations between temperature, pressure, compositions and quantities of phases at equilibrium. Phase diagrams allows to predict phase transformations which occur during temperature change (e.g. upon cooling).
All available thermodynamic and phase diagram data of the Mg–Ge and Mg–Pb binary systems, and the Mg–Ge–Pb ternary system have been critically evaluated and all reliable data have been simultaneously optimized to obtain one set of model parameters for the Gibbs energies of the liquid and all solid phases as functions of composition and temperature.
The invariant points determine the topology of the phase diagram: Figure 30-16: Construct the rest of the Eutectic-type phase diagram by connecting the lines to the appropriate melting points. Figure 30-17: Construct the rest of Peritectic-type phase diagram, on the left a rule for all phase diagrams is illustrated--the ``lines'' must ...
As may be noted, point C lies within the Liquid phase field. Therefore, only the liquid phase is present; its composition is 55 wt% Ag-45 wt% Cu. (d) The Mg-Pb phase diagram (Figure 9.20) is shown below; the point labeled "D" represents the 30 w t% Pb-70 wt% Mg composition at 425 °C.
Download scientific diagram | The phase diagram of the binary system, Pb–Mg. from publication: Eutectic Na–Tl and Pb–Mg alloys as liquid-metal coolants for fast nuclear reactors | The liquid ...
This video explains the Pb-Sn phase diagramFor further reading: https://www.physicsforums.com/threads/sn-pb-phase-diagram.281790/
Abstract. The liquidus curve for the Mg-Pb system was redetermined, and its slope was used to assess phase transformations in the β' + Pb-rich liquid phase field. A peritectic reaction occurred at 538.7 °C when Mg 2 Pb decomposed into a Mg-rich liquid and β' (a Mg-Pb compound containing 35.15 at. pct Pb, or 0.3515 N Pb, the atomic fraction of Pb) which melted congruently at 548.5 °C, confirming the work of Eldridge et al. [1] When β' was cooled, a series of reactions occurred with the ...
![Phase transformations and phase diagrams [SubsTech]](https://www.substech.com/dokuwiki/lib/exe/fetch.php?w=&h=&cache=cache&media=eutectic.png)









0 Response to "37 pb mg phase diagram"
Post a Comment