38 democritus atom model diagram
Democritus atomic theory is the ancient theory that describes the nature of matter in terms of atoms. According to Democritus (99-55 BC), atoms are infinite in number and eternal. Figure 01: Democritus We cannot create them, and the composition of atoms in a substance determines the qualities of that substance. shows changes to the model of the atom. Democritus. Diagram of Democritus model. John Dalton. Diagram of Dalton's model. How is Dalton's model the same as.
Democritus said that everything is made up into tiny bits, which are called atoms. These atoms are indestructible. He said that different shapes of atoms gave them different properties. For example, he said that things that tasted sweet were made of round atoms; whereas, things that tasted bitter were made of sharp atoms.
Democritus atom model diagram
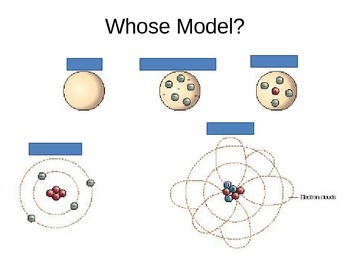
Democritus Model. Aristotle Model. Dalton model of the atom. Thomson's Model. Rutherford model of the atom. Bohr Model. Modern Cloud Model. ... Start studying atom diagrams. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Home Browse. Browse. Languages. English French German Latin Spanish View all. Learn democritus atomic model with free interactive flashcards. Choose from 500 different sets of democritus atomic model flashcards on Quizlet. Democritus and Aristotle greatest difference where their views with atoms.Democritus believed that the atom did exist and was the smallest unit of matter. Aristotle believe that there could be no the existence of the atom.Aristotle was incorrect , Democritus. they both developed their theory around 400 and 300 B.C in the era of ancient Greek ...
Democritus atom model diagram. Atomic model Model diagram Model Description 1. Democritus ... Based on the quantum mechanical model of the atom shown, fill in the blanks. 29. Write each isotope below in symbolic notation. Use the periodic table to determine the atomic number of each isotope. 1) neon-22 Aug 16, 2021 · Atomic Model of Democritus, Philosophical Atomism. The idea that matter is made up of separate units is very old. It appeared in many ancient cultures like Greece and India. The word " atom " comes from ancient Greek. ἄτομος, which means "indivisible", was invented by the pre-Socratic Greek philosopher Leucippus and his disciple the ... Oct 10, 2008 · Democritus was not discovered the atom; he proposed and accepted intutively the notion of atom at approx. 400 bc. 2. Now the founder of atomism is considered Leucippus, precursor and teacher of ... In short, this atomic model implied that all atoms were unstable. Diagram of an electron dropping from a higher orbital to a lower one and emitting a photon. Image Credit: Wikicommons The model...
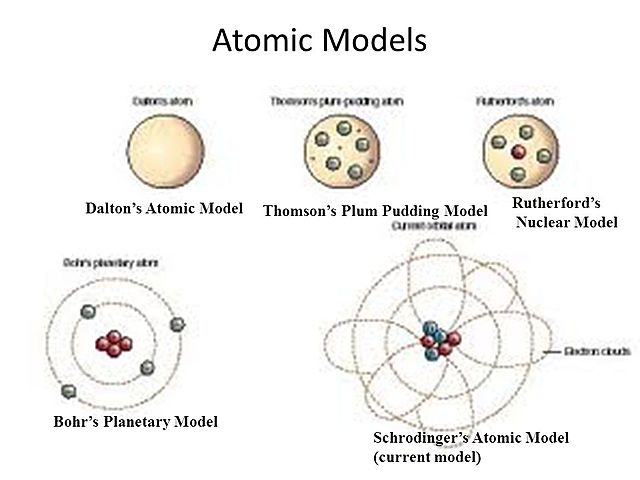
The diagrams in the timeline below are how each of the scientists thought an atom looked like. Each diagram was widely accepted until a newer, modern, and more realistic diagram came about. Originally, Democritus introduced the idea of the atom in Greece around 460 BC. What is the contribution of Democritus in atomic structure? Democritus was a central figure in the development of the atomic theory of the universe. He theorized that all material bodies are made up of indivisibly small " atoms .". Aristotle famously rejected atomism in On Generation and Corruption. Democritus was not able to describe atomic model in detail. On his theory, Democritus only stated that atoms are in the solid form in the void sphare. We can not describe the internal structure of... Democritus and the Atom Even though Democritus was the first to use the word atom he wasn't recognized for it and never had a atomic model or theory. Democritus' idea and use of the word "Atom" was the first step to building the foundation of chemistry with the atom thousands of years later!
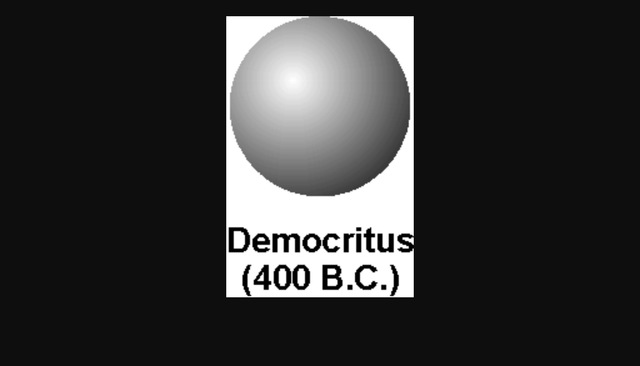
DEMOCRITUS. Lived from: 460-370 BC. Put forward atomic model in: 442 BC. Description of his model: Democritus’s model stated that matter consists of invisible particles called atoms and a void (empty space). He stated that atoms are indestructible and unchangeable. Also that they are homogenous, meaning they have no internal structure. Democritus Atom Model Diagram 15.08.2018 2 Comments The idea of atoms was invented by two Greek philosophers, Democritus and Leucippus in the fifth century BC. The Greek word ατoμoν (atom) means indivisible. B.C. - Democritus thought matter could not be John Dalton. • -Dalton proposed a modern atomic model Bohr - Rutherford diagrams. • Putting all. Democritus' atom: a long-forgotten model. For Aristotle the atomism of Democritus contradicted the concept of substance, in which the proportion of the elements (earth, air, water and fire) had to be maintained at all costs, no matter how small the fraction of it was. The substance for Aristotle is intrinsically continuous. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. This conclusion helped him propose 'Rutherford's Atomic Model'. According to his atom diagram, the atom has a small, positively charged nucleus in center. This nucleus carries the entire mass of the atom.
The idea of atoms was invented by two Greek philosophers, Democritus and Leucippus ... For example, an atomic model represents what the structure of an atom ...
Dalton expanded upon the ideas of Democritus, drawing a circle as the atom model. Dalton proposed that atoms are the smallest component of all molecules believing they combine in simple ratios. Dalton believed that atoms could not be destroyed.
Dec 15, 2021 · Democritus (460-370 BC) was a Greek philosopher who was the first to use the word "atom" (atomos means "indivisible and unbreakable" in Greek) to describe a small particle or substance. He created the first atomic model of a round sphere with electrons, protons and neutrons.
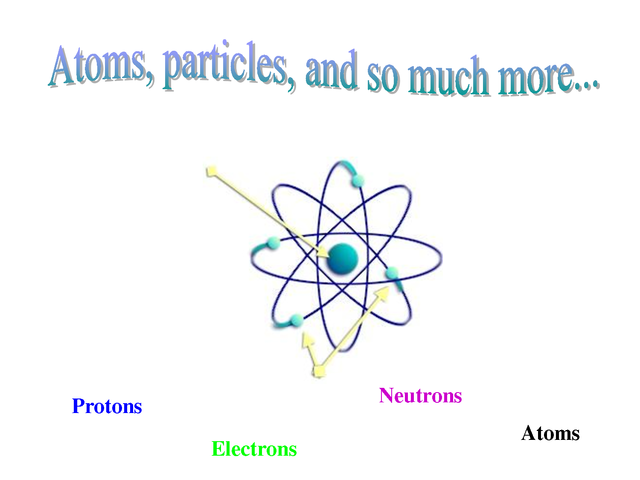
Key Takeaways: Model of the Atom. An atom is a building block of matter that cannot be broken apart using any chemical means. Nuclear reactions can alter atoms. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Protons and neutrons form the atomic nucleus.
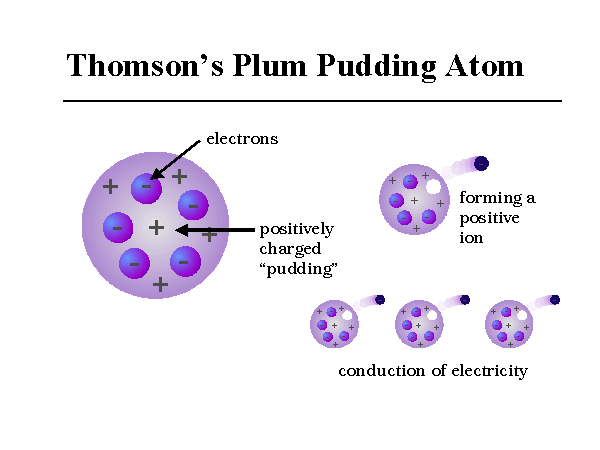
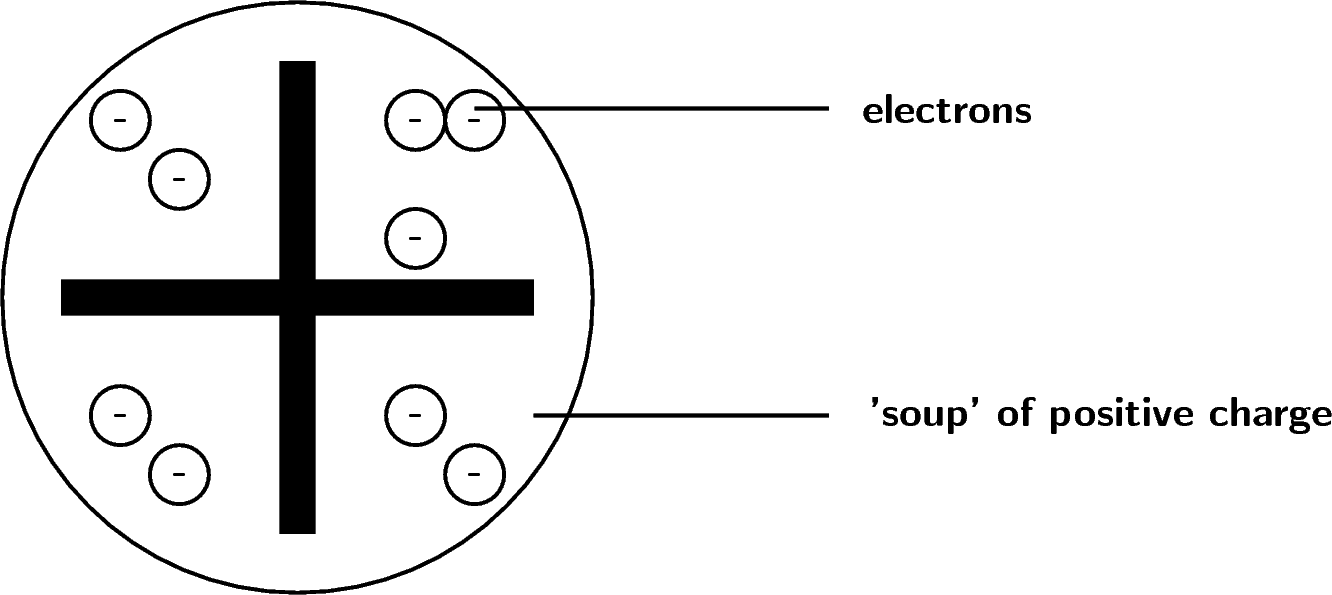
Information Atomic Model Analogy In 1897, the English scientist named J.J. Thomson provided the first hint that an atom is made of even smaller particles. He discovered the presence of a negative particle in the atom - the electron. He proposed a model of the atom that is sometimes called the "Plum Pudding" model.

it is apparent that this wanderer had experienced a meteoric rise and moved in powerful circles.. Bruno's cosmology:The universe is then one, infinite, immobile.... It is not capable of comprehension and therefore is endless and limitless
Models of the Atom: a Historical Perspective. Aristotle ... 400 B.C. - Democritus thought matter ... Putting all this together, we get B-R diagrams.1 page
Democritus's Atomic Theory is the foundation for all of chemistry and is incredibly relevant today. This is where the chemistry Modeling Instruction curriculum starts. Democritus made the observationthat if you break a rock into tiny pieces, those pieces are still made of rock.
The atoms of the soul were considered to be particularly fine. Democritus developed his atomic philosophy as a middle ground between two opposing Greek theories ...
John Dalton was the first to adapt Democritus' theory into the first modern atomic model. JOHN DALTON'S ATOMIC MODEL: 1. All matter consists of tiny particles ...5 pages

“Fashion is part of the daily air and it changes all the time, with all the events. You can even see the approaching of a revolution in clothes. You can see and feel everything in clothes.†—Diana Vreeland
This model has some good ideas in it, but overall it has some problems. The key (and not incorrect points) of this model are: The atom is made of protons, neutrons and electrons. Most of the space ...
Democritus did not realize that combinations of some types of atoms were sufficient to explain all the diversity of matter. On the contrary, the philosopher thought that the atom of the grains of sand was unique in sand. The same happened with wood and any other substance. Each had its own type of atom. In conclusion, for Democritus, the atom was the smallest possible fraction of each substance. Furthermore, the atom was solid and had no internal structure. The atoms of different materials can differ in size, shape, mass, giving the characteristics of that material. Among the conglomeration of atoms that make up any material, there is nothing but emptiness. Democritus, of course, lacked the experimental means to verify these claims. Nor two of the most prestigious Greek philosophers: Aristotle and Plato, who did not share these ideas about the atom. On the contrary, Aristotle and Plato supported Empedocles’ theory, which establishes four basic elements: earth, air, water and fire as...
Dalton's atomic model sets up the building blocks for others to improve on. Though some of his conclusions were incorrect, his contributions were vital. He defined an atom as the smallest indivisible particle.. John Dalton. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.
Answer: What was wrong with Democritus' atomic theory? Not a lot really. It has been wholly vindicated by the mathematical structure of elementary particle physics. It was actually the model of Leucippus, his teacher, but we only know of it from the elaborated version of Democritus (the elabora...
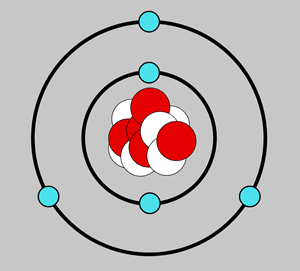
Atomic Model: Timeline • • Rutherford Democritus Thompson Bohr Chadwick Schrodinger Dalton. Bohr Model Bohr's theory begins with Rutherford's picture of an atom as a nucleus surrounded by electrons moving in circular orbits. In his theory, Bohr made a number of assumptions and combined the new quantum ideas of Planck and Einstein with the ...
Ancient History of the Atom. Democritus (460-370 BC), a Greek philosopher, was the first person to use the word atom or atomos (in Greek), which means indivisible or unbreakable, to describe the smallest particle of any substance. He believed that atoms were too small to be seen.
May 15, 2017 · 1. Democritus was not able to describe atomic model in detail. On his theory, Democritus only stated that atoms are in the solid form in the void sphare. We can not describe the internal structure of the atom itself. We now know that Atoms consist of 3 parts which are proton, neutron and electron. 2.
Democritus's _____7. Whose model determined that an atom's positive charge is concentrated in the atom's center? a. Rutherford's c. Democritus's ... Unlike the modern model of the atom, Bohr's model states that a. electrons move in set paths around the nucleus of an atom. ... Use the diagram to the right to answer question 1. _____1 ...
Democritus Atom Model Diagram. Democritus was an Ancient Greek pre-Socratic philosopher primarily remembered today for his formulation of an atomic. This is due to his theory of universe that is made up of tiny “atoms”, which bears .. Democritus' model of an atom was one of an intert solid that. In this lesson, we will review the ...
Democritus and Aristotle greatest difference where their views with atoms.Democritus believed that the atom did exist and was the smallest unit of matter. Aristotle believe that there could be no the existence of the atom.Aristotle was incorrect , Democritus. they both developed their theory around 400 and 300 B.C in the era of ancient Greek ...
Learn democritus atomic model with free interactive flashcards. Choose from 500 different sets of democritus atomic model flashcards on Quizlet.
Democritus Model. Aristotle Model. Dalton model of the atom. Thomson's Model. Rutherford model of the atom. Bohr Model. Modern Cloud Model. ... Start studying atom diagrams. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Home Browse. Browse. Languages. English French German Latin Spanish View all.




















0 Response to "38 democritus atom model diagram"
Post a Comment