37 adiabatic process pv diagram
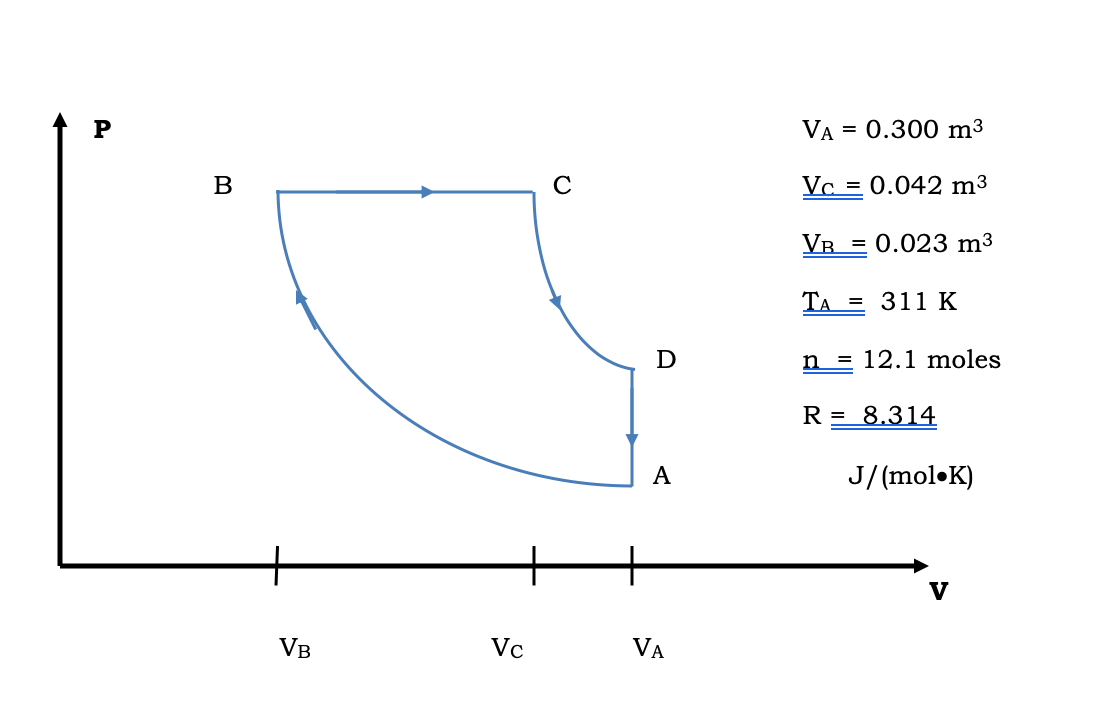
For an adiabatic process of ideal gas equation we have $PV^{\gamma} = K$ Where Calculate the work done in the process? Given $4^{1.4} =6.96$ Solution For an adiabatic Process $PV Question 2 Consider a P-V diagram in which the path followed by one mole of perfect gas in a cylindrical... This is just for fun, not homework. So have a go and see how you stack up against the community! [View Poll](https://www.reddit.com/poll/nnv4t5)
Hello, so I'm a little confused about the relationship between the three concepts. Here is what I know: - during an adiabatic process, there is no heat exchange. Therefore the change of energy is due to work (deltaE=q+w) - heat refers to a transfer in energy which is dependent on temperature because Q=mc∆T Here's what I don't understand: In an adiabatic process, Q=0 which would mean that ∆T should be 0 (since it's a closed system and m and c aren't changing). However, temperature in an adiab...

Adiabatic process pv diagram
Hi! I’m currently working on a project based on the automarizarion of evaporative air coolers. I’m trying to create a mathematical model that can help me interpret a psychrometric chart/ get the results I’d get from one using equations. Has anybody done this before? Or does anyone have any tips? Thanks! * ***Title:*** Twenty-five Years of Progress in "adiabatic" Adsorption Processes * ***Authors:*** Robert T. Cassidy, Ervine S. Holmes * ***Link:*** [https://books.google.cz/books/about/Twenty\_five\_Years\_of\_Progress\_in\_adiabat.html?id=6TmaYgEACAAJ&redir\_esc=y](https://books.google.cz/books/about/Twenty_five_Years_of_Progress_in_adiabat.html?id=6TmaYgEACAAJ&redir_esc=y) So there are 2 processes for 1kg of Water. 1st is adiabatic and second is isobar. Everything that is given: p1= 0.1MPa , p2 = p3 = 1MPa, T1 = 100C and T3 = 400C.. how do I calculate T2 without calculating the Isentropic expontent Kappa (or K whatever you like). I can see the Volume V3 from some given Table but I would need V2 how do I get it?
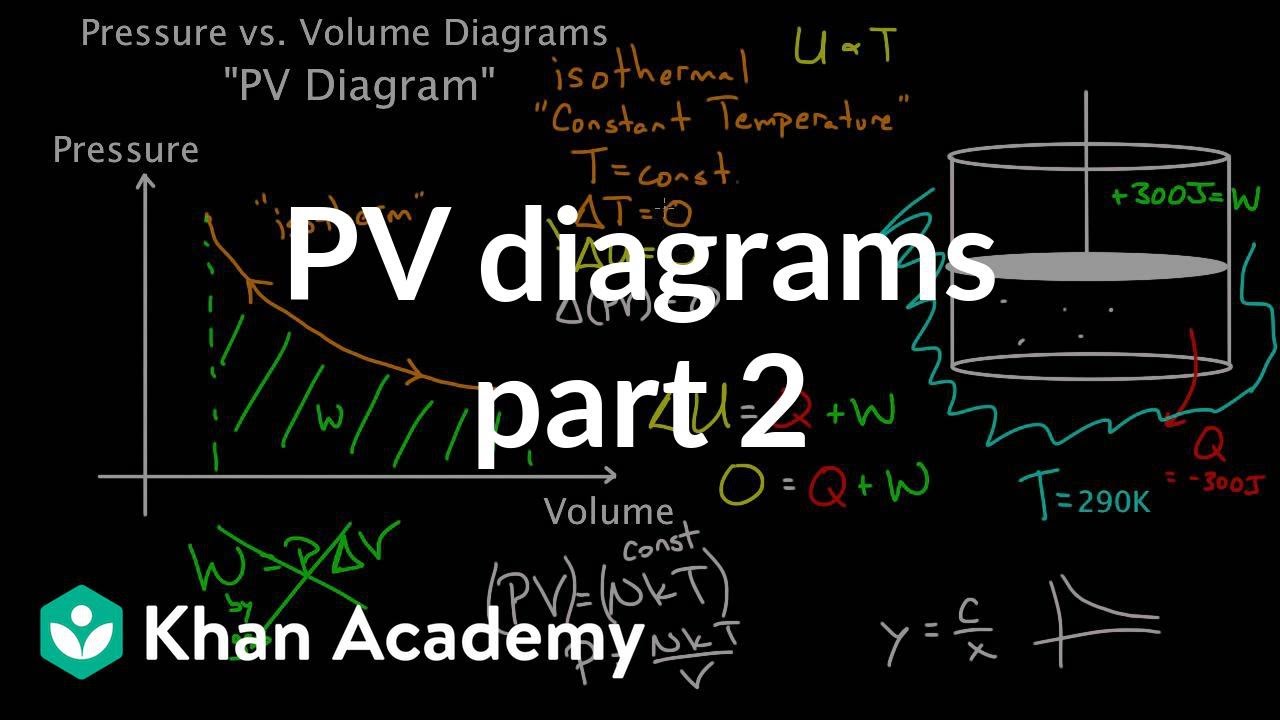
Adiabatic process pv diagram. The lesson begins with the slope of an isothermal curve on PV diagram with proper graphical illustrations. Then explanation moves on to slope of adiabatic curves on PV diagram with useful mathematical equations and explanation. This is just for fun, not homework. So have a go and see how you stack up against the community! [View Poll](https://www.reddit.com/poll/nmg59g) I am currently reviewing some Thermodynamics and as it has been quite a few years since my university days, I am having trouble understanding a few things. In particular I am wondering about the difference between reversible and adiabatic processes and the various changes of entropy that occur. I've read several online sources but some of the explanations don't seem to quite line up with each other. My guess is that there are various definitions being used and some are more simplified that othe... Adiabatic. Summary Of Processes. Lesson 42c: PV Diagrams. From the last section, you were probably wondering what happens when we do something like add heat. to a sealed cylinder.
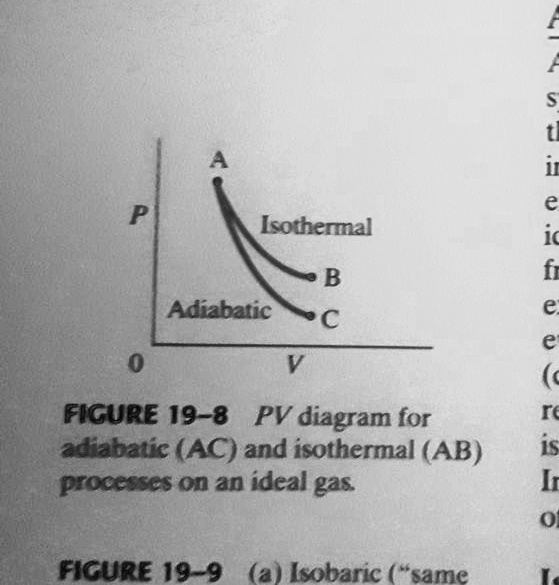
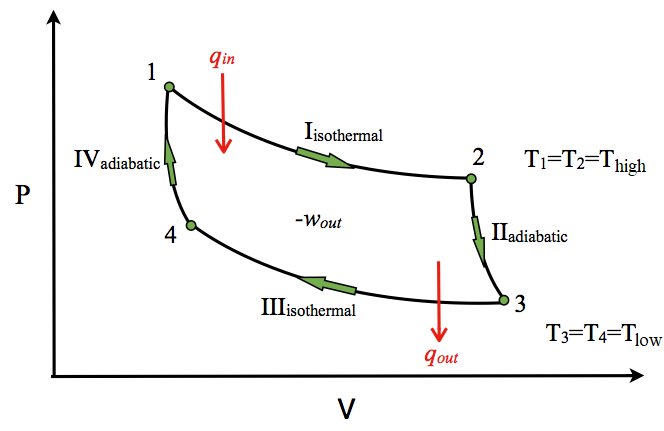
The classical Carnot heat engine. Category. v. t. e. In thermodynamics, an adiabatic process (Greek: adiábatos, "impassable") is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment. The adiabatic process represents a state change within a system where there is no heat exchanged. Therefore, Q is equal to 0 and it is known that the change pv diagram, pressure, volume, adiabatic, process, adiabat, isometric, isothermal, isotherm, isobaric, isobar, isochoric, work, heat, first law of... PV diagrams clearly illustrate that the work done depends on the path taken and not just the endpoints. This path dependence is seen in Figure 7a, where Both isothermal and adiabatic processes such as shown in Figure 8 are reversible in principle. A reversible process is one in which both the system... https://ibb.co/YbwkCsB This seems basic but it has been annoying me alot, why can we write dU the way it is and not have the first term, TdS be zero? Is it because it is not a reversible process and hence dQ<TdS ? But it seems to me that this quasi-static process has no friction involved and hence it is reversible. What am I missing?
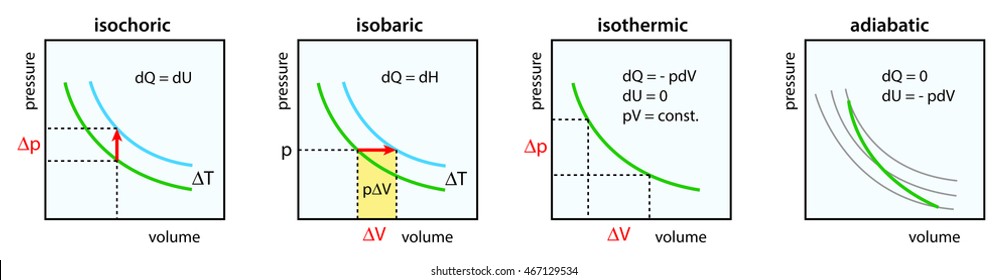
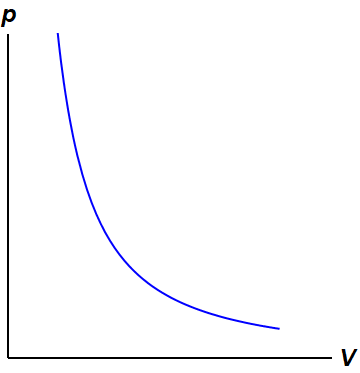
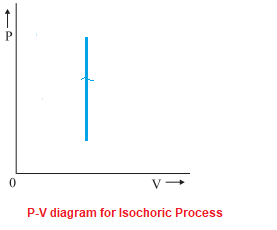
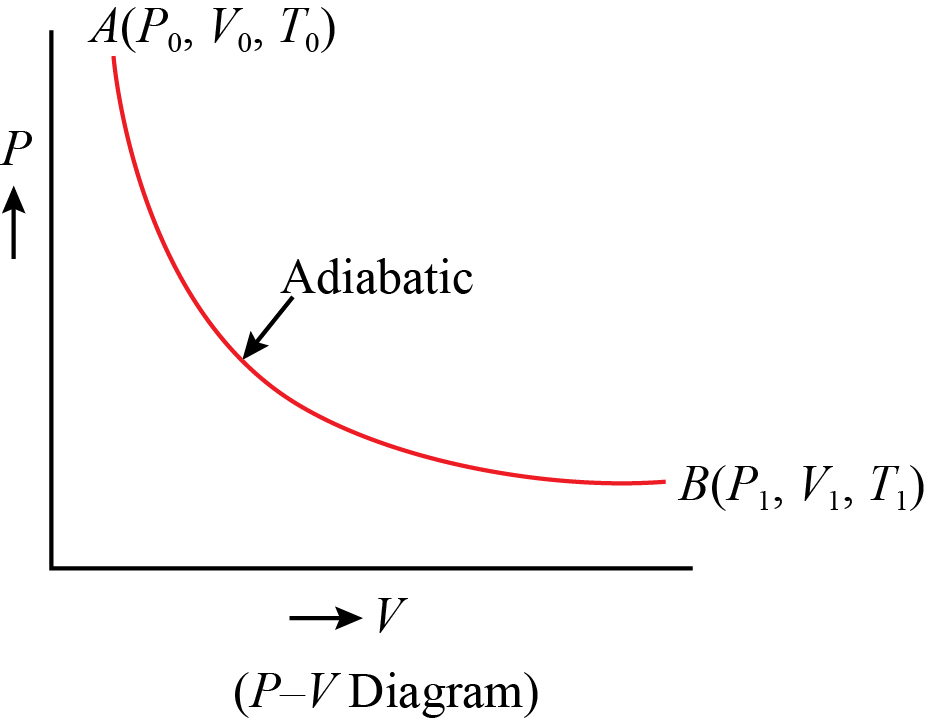
Adiabatic process is a process in which there is no exchange of heat takes place from the working substance to the surrounding during its expansion or In the P-V diagram that is shown above, a gas in the piston cylinder assembly is heated adiabatically (i.e. not heat leaves or enters into the gas. CPS question This pV-diagram shows two ways to take a system from state a (at lower left) to state c (at upper right) Thermodynamic Processes: •Adiabatic: no heat transfer (by insulation or by very fast process). Q=0 → U2 - U1 = -W •Isochoric: constant volume process (no work done). PV diagrams - part 2: Isothermal, isometric, adiabatic processes | MCAT | Khan Academy. Learn Adiabatic Process topic of Physics in details explained by subject experts on vedantu.com. Register free for online tutoring session to clear your Adiabatic- Reversible and Irreversible Process. There are four types of process in a thermodynamic system, which are shown via an image below
**Process Flow Diagram:** https://preview.redd.it/pwp6kssnldc81.jpg?width=5313&format=pjpg&auto=webp&s=3cf24795d5ca2644f6cd12d5b11bc80da6756a5d ​ This system is designed to perform a baseline ore processing chain through: 1. Universal Macerator 2. Ore Washing Plant 3. Thermal Centrifuge 4. Universal Macerator 5. Combine partial byproducts into full dusts 6. Output final products / bypass any materials that are not designated for processing Additional system functiona...
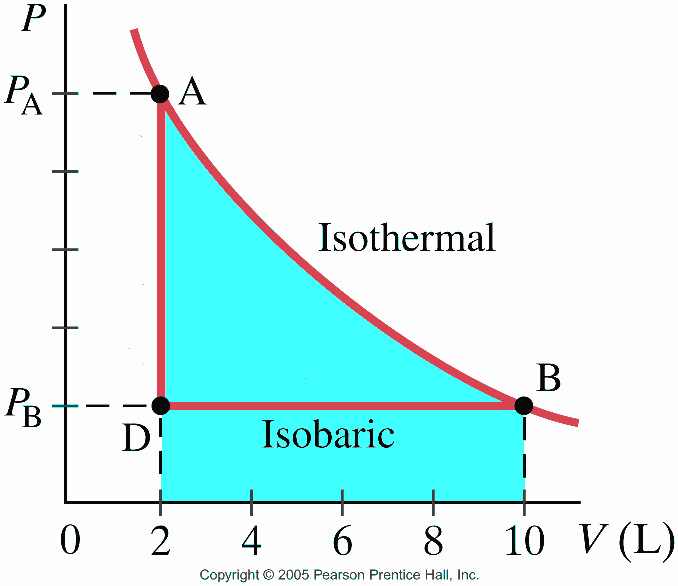
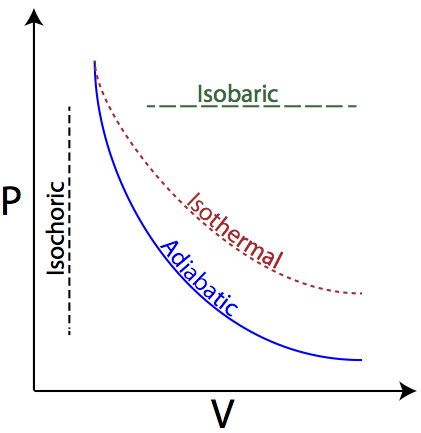
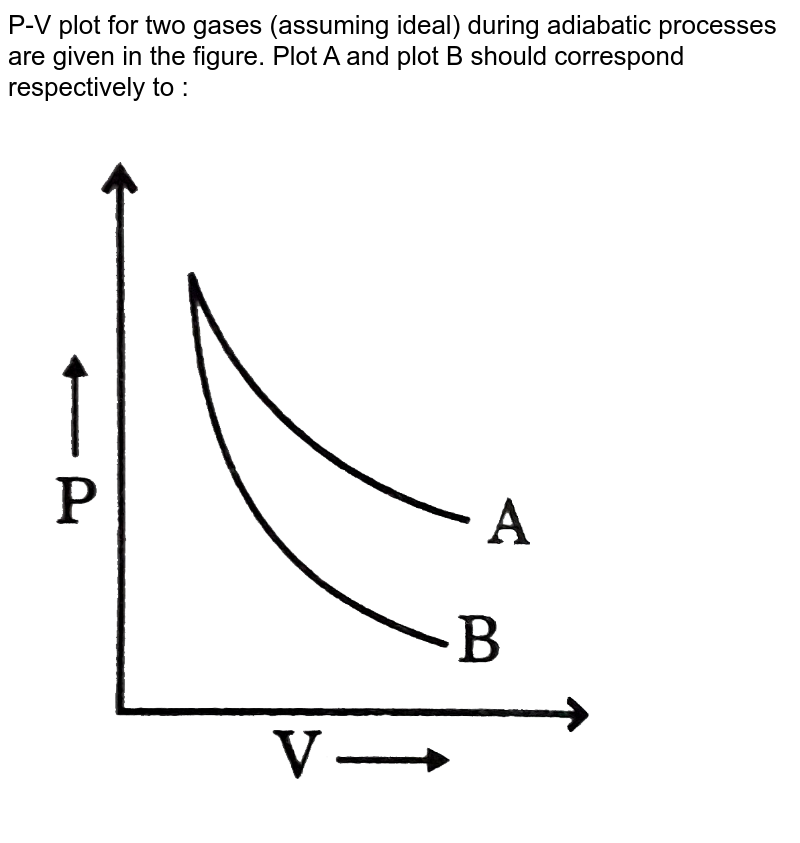
diagram, only adiabatic and isotherm processes have asymptotic shapes. An example case for the curves for the two processes is shown here In that case, PV is constant only for Isothermal process or in a process where there is no temperature change whatsoever as the process proceeds.
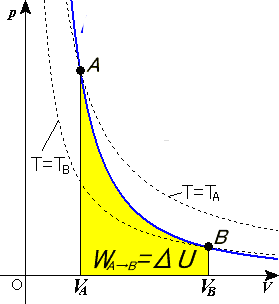
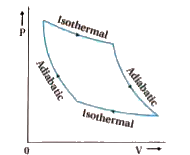
Assuming this PV diagram describes a process involving an ideal gas, we can deduce the following If we attempt to compute the entropy change for c→d by finding a point f on the PV diagram such that c→f is adiabatic and f→d is isothermal, we find that we have constructed a Carnot...
Another interesting adiabatic process is the free expansion of a gas. This equation is the condition that must be obeyed by an ideal gas in a quasi-static adiabatic process.
This system is designed to perform a baseline ore processing chain through: 1. Universal Macerator 2. Ore Washing Plant 3. Thermal Centrifuge 4. Universal Macerator 5. Combine partial byproducts into full dusts 6. Output final products / bypass any materials that are not designated for processing Additional system functionality: 1. Filter and Macerate selected non-ore materials 2. Filter and process specific ores through mercury & sodium persulfate chemical baths to boost select byproduct...
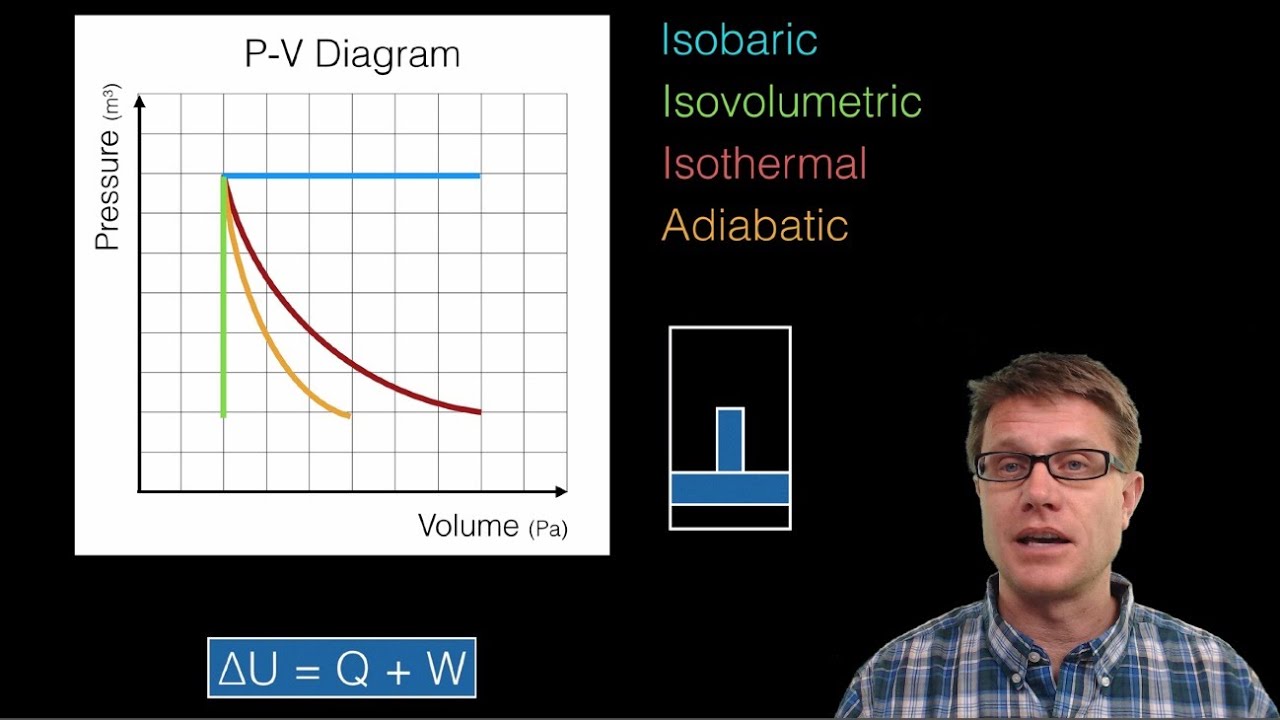
Slope of Isothermal and Adiabatic Process on PV Diagram and key points about different slopes in Hindi by D Verma Sir join me ... ... PV diagrams. It explains how to calculate the work done by a gas for an isobaric process, isochoric process, isothermal process
Visit us (http://www.khanacademy.org/science/healthcare-and-medicine) for health and medicine content or (http://www.khanacademy.org/test-prep/mcat) for MCAT related content. These videos do not provide medical advice and are for informational purposes only. The videos are not intended to be a...
Experiment 3A: Adiabatic Gas Law Experiment 3B: Work Done by an Adiabatic Process. To show that PV/T = nR for an ideal gas and determine the value of R. Physical Principles. 3. Quickly raise the piston in an adiabatic expansion and hold up at the top of the stroke (Stroke 3 to 6 in the diagram).
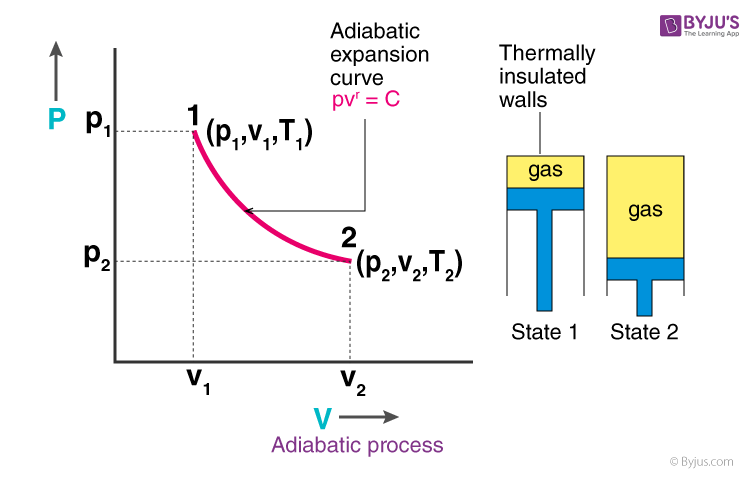
Adiabatic. Figure 1: The purple line is an example of an adiabat on a PV diagram. Adiabatic refers to a process in which no heat is transferred into or out of a system , and the change in internal energy is only done by work .
In adiabatic processes where there is no energy transfer in the form of heat, the following relation is derived from Eqs. An adiabatic process is a reversible constant entropy process for an ideal gas without heat transfer, following the relationship. A p - V diagram of the cycle is depicted in Fig.
Is it possible (at least with the tools of a first year student)?
File:Adiabatic-process-in-p-V-diagram.svg. From Wikimedia Commons, the free media repository. Jump to navigation Jump to search. English: Adiabatic process in p-V-diagram. Date. 23 November 2019.
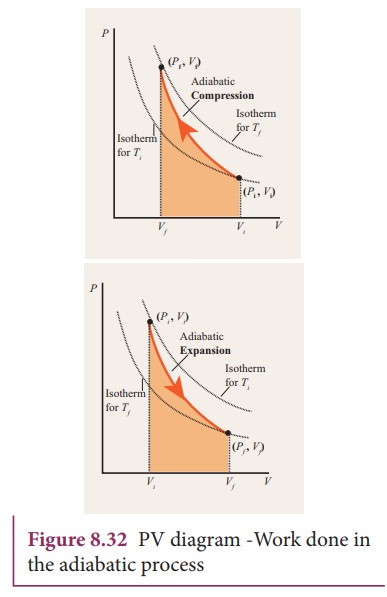
PV diagram for adiabatic expansion and adiabatic compressior. (d) Isochoric process : (a) increased pressure and.
Isothermal process ,Adiabatic process, Isobaric process, Solved Example Problems for Thermodynamic Processes. From the PV diagram the area under the curve AB is more, implying that the work done in isobaric process is highest and work done in adiabatic process is least.
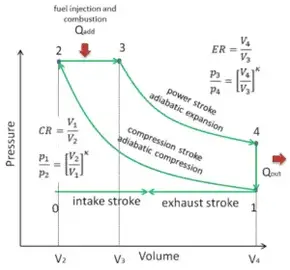
An adiabatic process is one in which no heat is gained or lost by the system. The first law of thermodynamics with Q=0 shows that all the change in internal energy is in the form of work done. This puts a constraint on the heat engine process leading to the adiabatic condition shown below.
Homework Statement Give a definition of an adiabatic process. Derive an expression for the work performed by n mol of an ideal gas undergoing an... Thermodynamics (Adiabatic PV Diagram). Thread starter alexgmcm. Start date Apr 8, 2010.
In my new job I need to create flow diagrams of various manufacturing processes. These diagrams will usually contain material flow (good + rejects), data flow, etc. I have been using MS Visio to make the diagrams so far, but I feel like I'm always fighting to get the software to do what I want. There are a couple features that I think it's really lacking in, namely: * There is no infinite zoom feature! I haven't been able to find this in any "flow diagram" software. If you're confused what I me...
Another interesting adiabatic process is the free expansion of a gas. (Figure) shows a gas confined by a membrane to one side of a two-compartment, thermally insulated container. A reversible adiabatic expansion of an ideal gas is represented on the pV diagram of (Figure).
2.11 Adiabatic changes - Poisson equations. The next sections will discuss the theoretical background for describing experiments performed under various specific boundary conditions. These boundary conditions are often a significant simplification for the real experimental boundary conditions but...
Process functions: Work · Heat Specific heat capacity c= Compressibility β = − 1 V Thermal expansion α = 1 Property database Compressibility factor From Wikipedia, the free encyclopedia The compressibility factor (Z), also known as the compression factor, is a useful thermodynamic property for modifying the ideal
Question: Suppose 2.00 mol of a monatomic ideal gas (cV = 3/2R) is expanded adiabatically and reversibly from a temperature T = 300 K, where the volume of the system is 20.0 L, to a volume of 60.0 L. Calculate the final temperature of the gas, the work done on the gas, and the energy and enthalpy changes. ​ I calculated the final temperature by using the following equation: T1V1\^(cp/cv)-1 = T2V2\^(cp/cv)-1 In which I got Tfinal = 144 K (I checked the answer key and this is corre...
PV during a polytropic process. > In an adiabatic change, the pressure and temperature of a monoatomic gas are related with relation as P∝TC, where C is equal to The process BC is adiabatic. The temperatures at A, B and C are 400K, 800K and 600K respectively.
Hello, so I'm a little confused about the relationship between the three concepts. Here is what I know: - during an adiabatic process, there is no heat exchange. Therefore the change of energy is due to work (deltaE=q+w) - heat refers to a transfer in energy which is dependent on temperature because Q=mc∆T Here's what I don't understand: In an adiabatic process, Q=0 which would mean that ∆T should be 0 (since it's a closed system and m and c aren't changing). However, temperature in an adiab...
Can someone help explain to me whats the difference between W=-PV and W = PV, when do I know when to have the negative in front? Also, can someone tell me how I can tell if work is being done by the system vs work being done on the system and the correct sign conventions?? Thanks!
In non isolated systems where there is no adiabatic process, $PV$ is constant. But the graph gets steeper in adiabatic process because of the $\gamma$ over the $V$.
Adiabatic process. Computational example. Carnot cycle. Combined gas law calculator is a great tool to deal with problems related to the most common transformations of gases. Read about isobaric, isochoric, isothermal, and adiabatic processes of ideal gases...
The pV-diagram for the isothermal process is shown below. Now we consider an adiabatic process, with the same starting conditions and the same final. Sketch the adiabatic processes on the p-V diagram below and compute the final temperature. (Ignore.
I am currently reviewing some Thermodynamics and as it has been quite a few years since my university days, I am having trouble understanding a few things. In particular I am wondering about the difference between reversible and adiabatic processes and the various changes of entropy that occur. I've read several online sources but some of the explanations don't seem to quite line up with each other. My guess is that there are various definitions being used and some are more simplified that othe...
Academia.edu is a platform for academics to share research papers.
I'm somewhat familiar with augmenting real-life images to get a larger and more generalisable dataset for building a ML model. But when it comes to maps or engineering diagrams, how can I build a model such that it looks at the legend to know the existing elements and identify them in the image? Say, I have a map like [this image](https://lotusarise.com/wp-content/uploads/2021/03/agricultural-productivity-in-india-map.png), what model/tool should I use that can look at the legend, learn that th...
So there are 2 processes for 1kg of Water. 1st is adiabatic and second is isobar. Everything that is given: p1= 0.1MPa , p2 = p3 = 1MPa, T1 = 100C and T3 = 400C.. how do I calculate T2 without calculating the Isentropic expontent Kappa (or K whatever you like). I can see the Volume V3 from some given Table but I would need V2 how do I get it?
* ***Title:*** Twenty-five Years of Progress in "adiabatic" Adsorption Processes * ***Authors:*** Robert T. Cassidy, Ervine S. Holmes * ***Link:*** [https://books.google.cz/books/about/Twenty\_five\_Years\_of\_Progress\_in\_adiabat.html?id=6TmaYgEACAAJ&redir\_esc=y](https://books.google.cz/books/about/Twenty_five_Years_of_Progress_in_adiabat.html?id=6TmaYgEACAAJ&redir_esc=y)
Hi! I’m currently working on a project based on the automarizarion of evaporative air coolers. I’m trying to create a mathematical model that can help me interpret a psychrometric chart/ get the results I’d get from one using equations. Has anybody done this before? Or does anyone have any tips? Thanks!
















0 Response to "37 adiabatic process pv diagram"
Post a Comment