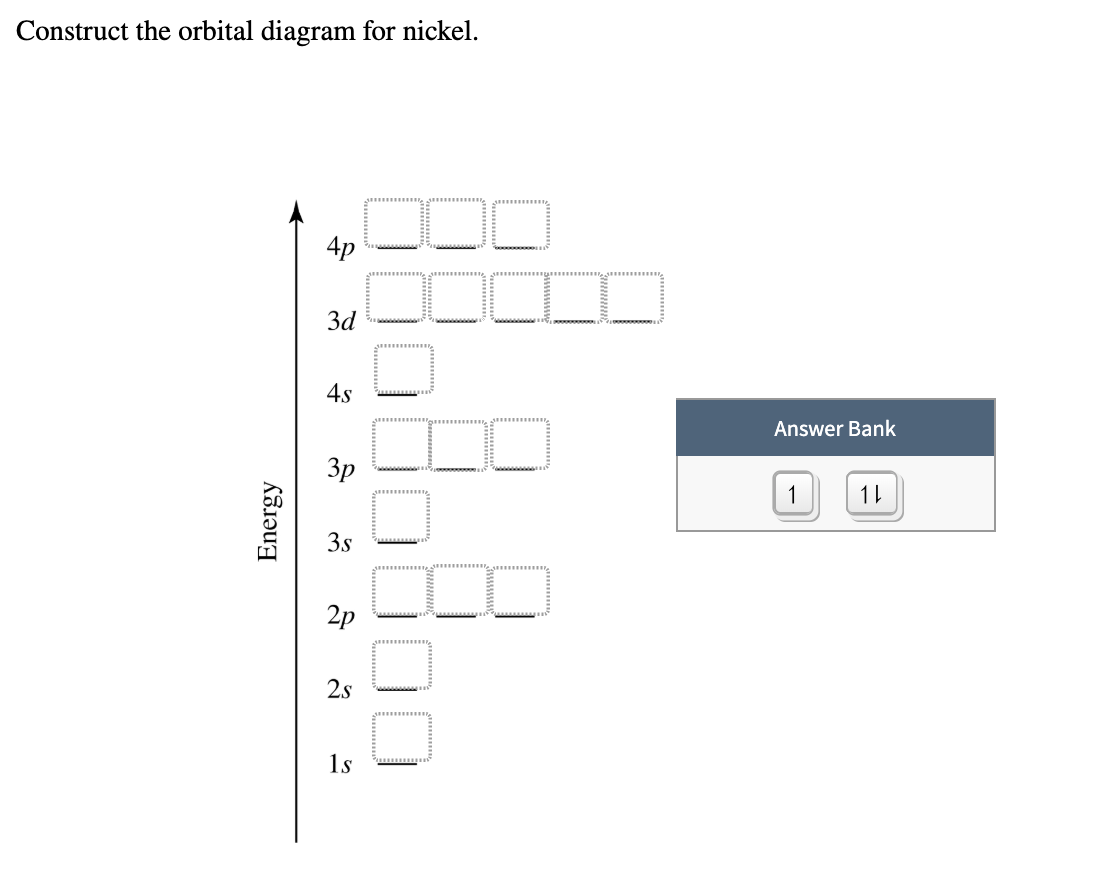
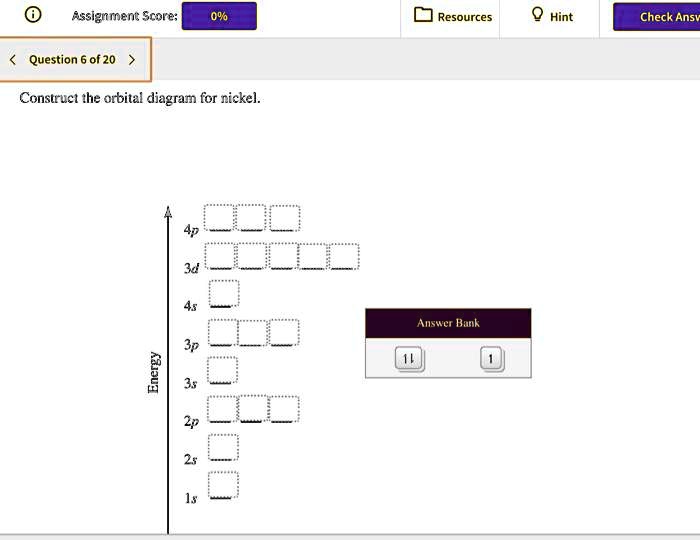
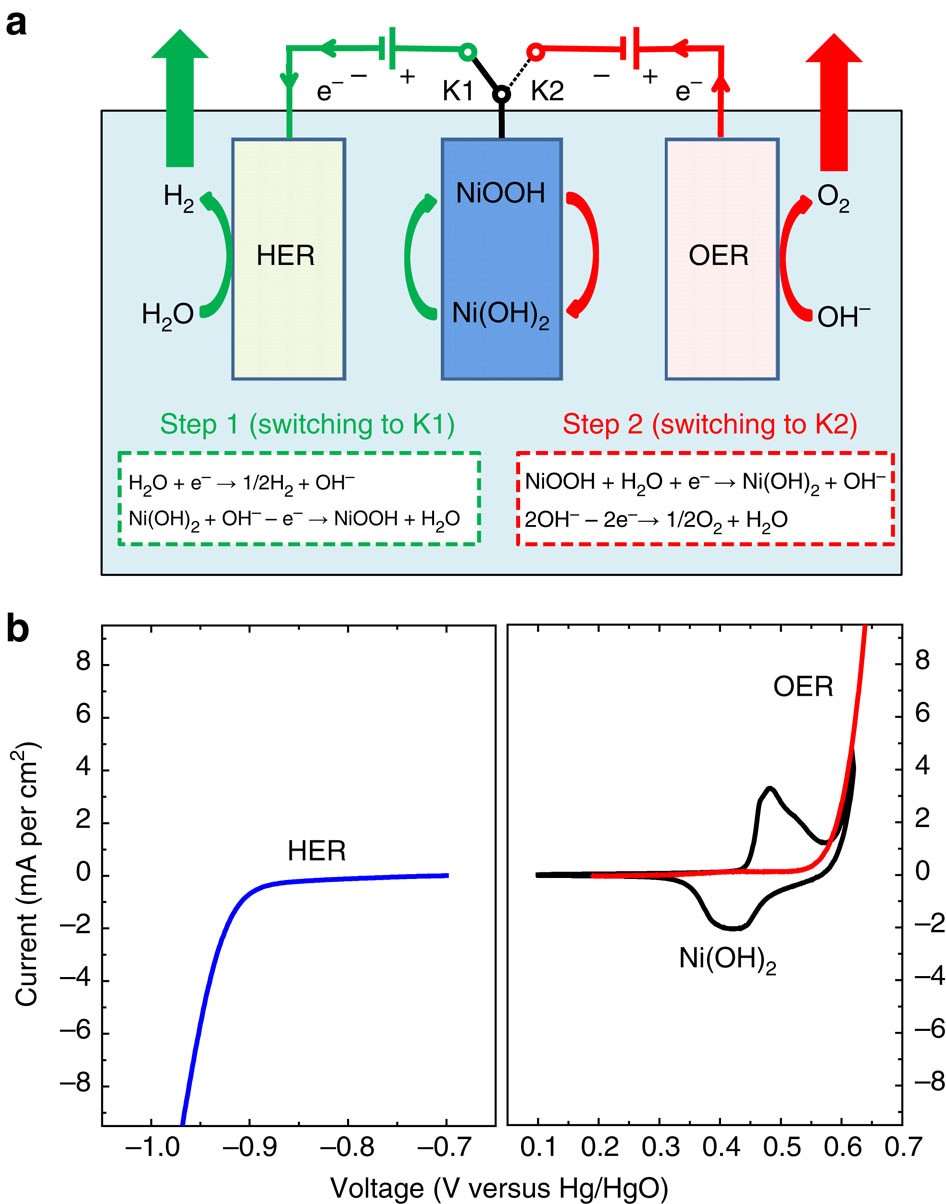
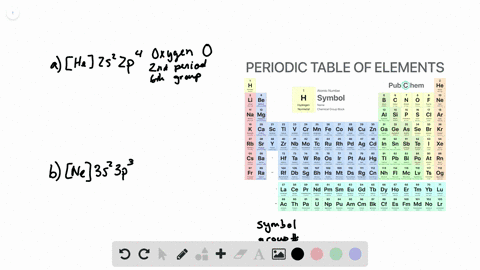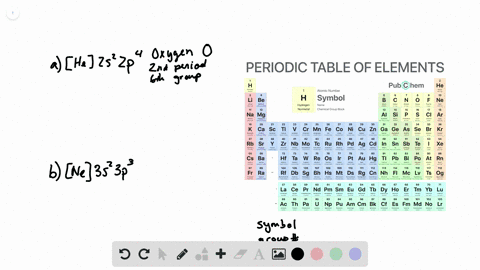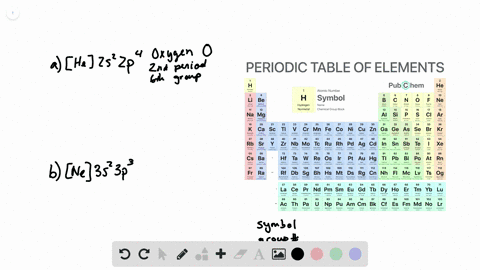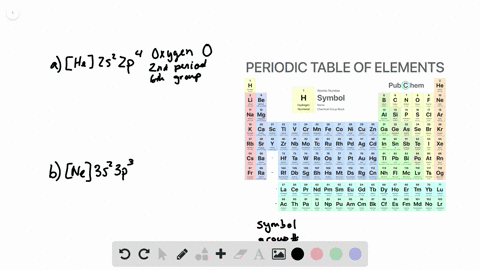
37 construct the orbital diagram for ni.
Construct The Orbital Diagram For Ni Answer to Construct the orbital diagram for Ni. Start by adding the appropriate subshells. For example, carbon is in the 2p block.1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. Modeling materials using density functional theory For Fe, Co, and Ni, the experimental values are 2.22, 1.72, and 0.61 Bohr-magnetons, respectively kittel and are usually good initial guesses. See Reference PhysRevB.56.15629 for a very thorough discussion of the determination of the magnetic properties of these metals with DFT. For a hydrogen atom, an initial guess of 1.0 Bohr-magnetons ...
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine

Construct the orbital diagram for ni.
Construct the orbital diagram for Ni. - HomeworkLib The atomic number of nickel is 28. So, there are 28 electrons in nickel atom. First start filling the electrons with lowest energy orbital and each orbital is singly occupied with one electron. And only two electrons with opposite spin can fit into an orbital. Thus, orbital diagram of nickel is as follow. Ans: 31 Add a comment Know the answer? Nickel Electron Configuration (Ni) with Orbital Diagram 26 Jan 2021 — Valence electrons are the electrons which are located in the outer shell or orbit. There are 28 electrons in the nickel in the 4 orbits and the ... Solved Construct the orbital diagram for Ni. | Chegg.com Question: Construct the orbital diagram for Ni. This problem has been solved! See the answer See the answer See the answer done loading. Construct the orbital diagram for Ni. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
Construct the orbital diagram for ni.. Moving beyond bimetallic-alloy to single-atom dimer atomic ... Nov 19, 2021 · Meanwhile, the Ni-Co/Co-Ni paths were fitted at a position of 2.55 Å with CNs of 0.7/0.6, directly indicating the existence of a significant amount of Ni-Co bonding dimer in the form of NiCo-N 6 ... Wiring Diagram Pictures - schematron.org Construct The Orbital Diagram For Ni. 03.06.2019 03.06.2019. 76 Plymouth Duster Dome Light Wiring Diagram ... 03.06.2019 03.06.2019. 2007 Suzuki Forenza Wiring Diagram For Keyless Entry. 03.06.2019 03.06.2019. Rochester Quadrajet Carburetor Vacuum Diagram. 03.06.2019 03.06.2019. Tls2-gd2 Wiring Diagram ... Vanadium Orbital Diagram; 92 Cadillac ... orbital notation for iron - Henchen Construction In an orbital (box) diagram a box represents each notation and an orbital diagram. Construct the orbital diagram for ni. Select the correct answer and click on the "Finish" button Check your score and answers at the end of the … Fe, or iron, has an atomic number of 26. Oxidation States, 2. Periodicities in the K 2 lightcurve of HP Librae Figure 1. Sections of the K2 lightcurve taken at 3 different times of observation before (top) and after (bottom) subtraction of the positive superhump. The variations between successive superhump cycles in the top panel are more than the amplitude of the orbital period, and as a result the superhump might still have significant residual contributions even after subtraction.
Orbital Diagrams.docx - Organizing Electrons Electrons are ... Then draw the orbital diagram for the given element. Element # protons # neutrons Orbital Diagram Be 4 5 N. 7 7 Ne 10 10 Na 11 11 P 15 16 S 16 16 Ca 20 28 Sc 21 24 Fe 26 30 Ni 28 30 Zn 30 34. Share this link with a friend: Copied! Other Related Materials. How to Draw Orbital Diagrams - YouTube Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... Nickel (Ni) - ChemicalAid Nickel (Ni) has an atomic mass of 28. Find out about its chemical and physical ... Orbital Diagram. Ni - Nickel - Orbital Diagram - Electron Configuration ... What is the orbital diagram for nickel? - Quora Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev...
Construct The Orbital Diagram For Ni Question: Construct the orbital diagram for Ni. Construct the orbital diagram for Ni. see more. Best answer. Atomic orbital diagrams are also known as electron-in-a-box diagrams. These are simplified diagrams of how electrons are arranged within the orbitals for a. Answer to Construct the orbital diagram for Ni. Start by adding the appropriate ... What is the orbital diagram for nickel? - Answers The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d8. In all of the cases, both up and down arrows are filled, with the exception of the 3d shell, where the last two are up ... Wiring Diagrams Free DOWNLOAD Wiring Diagrams Free. Close DOWNLOAD. Construct The Orbital Diagram For Ni. More Details . 76 Plymouth Duster Dome Light Wiring Diagram. More Details . 2003 Envoy 4.2 Liter Cooling Fan Wiring Diagram. More Details . Jvc Kd-g230 Wiring Diagram. More Details . Warwick Corvette Wiring Diagram. PDF Hybrid Molecular Orbitals - University of Illinois Urbana ... Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals 1. Begin with the Lewis structure. 2. Decide how many orbitals each atom needs to make its sigma bonds and to hold its non-bonding electrons. Draw the atomic and hybrid orbitals on on side of the page. 3. For each sigma bond, take a hybrid (or atomic) orbital from ...
QUESTION 24 Draw the orbital diagram for ... - Physical ... QUESTION 24 Draw the orbital diagram for nickel (Ni). How many unpaired electrons does Ni have and is Ni paramagnetic or diamagn. ... with the nitrogen atoms forming N2. Draw one of the resonance structures for N20 (one N is central). Include all lone pair electrons and any nonzero formal charges in your structure. eBook References draw ...
PDF MO Diagrams for More Complex Molecules • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11 ...
PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals?
Solved Construct the orbital diagram for nickel. Answer ... Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 90% (20 ratings) Transcribed image text: Construct the orbital diagram for nickel. Answer Bank 111 Energy.
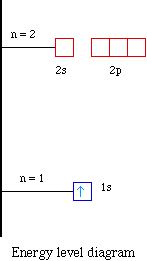
Orbital Diagrams Chemistry Tutorial - AUS-e-TUTE An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
Why is "Ni"("CN")_4^(2-) diamagnetic but "NiCl"_4^(2 ... Their blank d -splitting diagrams within the realm of crystal field theory are: [Ni(CN)4]2−: The d orbitals fill with 8 electrons, then, with a low spin configuration. You can see that an even number of d orbitals will get filled ( dyz,dxz,dz2,dxy) with an even number of 3d electrons. This gives rise to a diamagnetic configuration, as expected.
Electronegativity - Wikipedia The electronegativity of an atom changes depending on the hybridization of the orbital employed in bonding. Electrons in s orbitals are held more tightly than electrons in p orbitals. Hence, a bond to an atom that employs an sp x hybrid orbital for bonding will be more heavily polarized to that atom when the hybrid orbital has more s character.
Product selectivity of photocatalytic CO2 ... - ScienceDirect Jan 01, 2020 · For transition metal-based photocatalysts, the interaction between the transition metal d orbital and CO 2 molecular orbital is directly reflected in the adsorption energy. When the d-band center is low, the d orbital electron is bound. The interaction with the molecular orbital of CO 2 is weak.
PDF Simple Molecular Orbitals - Sigma and Pi Bonds in ... - CPP orbital diagram and that would force us to fill in the bonding sigma MO and the anti-bonding sigma-star MO. What we gain in the bonding sigma MO, we lose in the anti-bonding sigma-star MO. There is no advantage for two helium atoms to join together in a molecule, and so they remain as isolated atoms (note
Draw a molecular orbital diagram of N2 or O2 with magnetic ... Draw a molecular orbital diagram of ${N_2}$ or ${O_2}$ with magnetic behavior and bond order. Verified. 92.9k+ views. Hint: Generally the molecular orbital diagrams are used to understand the bonding of a diatomic molecule. You should know that molecular orbital diagrams are used to deduce magnetic properties of a molecule; they also help us to ...
What is the orbital diagram for nickel? - Quora Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: · Given the rules, the orbital diagram for Ni is:.
Dynamical Mean Field Studies of Infinite Layer Nickelates ... al. [21] use a four-orbital model: Ni d xyorbital, lanthanide d 3z 2 r orbital, lanthanide d xyorbital and interstitial sorbital. Local interaction is added on Ni-d x 2 y. The model is used to study the interplay between hybridization and correlation effects and to calculate the phase diagram. Gao et al. [22] construct a general four-orbital ...
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
PDF Electron Configuration Worksheet - Webs Draw the orbital diagrams for the following IONS. This will be the same orbital diagrams as a neutral atom except you've added or subtracted some arrows to represent the electrons that were added or subtracted.
Titanium oxide and chemical inhomogeneity in the atmosphere ... Jan 27, 2022 · Row 4: K p –V sys diagram showing the expected and observed orbital and radial velocities. In the case of different velocities, the observed velocity is indicated with an opaque line.
Construct the orbital diagram for Ni. - Clutch Prep Problem: Construct the orbital diagram for Ni. FREE Expert Solution Ni → atomic # 28 → 28 electrons. Ni will pass through 1s, 2s, 2p, 3s, 3p, 4s2, 3d. Following Aufbau principle (fill lowest energy first) and Hund's rule (half-filled first before totally filled) 82% (205 ratings)
Problem: Construct the orbital diagram for Ni. - Clutch Prep We're being asked to construct the orbital diagram for Ni. For that, we first need to determine the electron configuration of Ni. Recall that for a neutral element, Atomic number = # of protons = # of electrons. The atomic number of Ni is 28 and since it's a neutral element, this means Ni has 28 electrons. 95% (479 ratings)
Solved Construct the orbital diagram for Ni. | Chegg.com Question: Construct the orbital diagram for Ni. This problem has been solved! See the answer See the answer See the answer done loading. Construct the orbital diagram for Ni. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
Nickel Electron Configuration (Ni) with Orbital Diagram 26 Jan 2021 — Valence electrons are the electrons which are located in the outer shell or orbit. There are 28 electrons in the nickel in the 4 orbits and the ...
Construct the orbital diagram for Ni. - HomeworkLib The atomic number of nickel is 28. So, there are 28 electrons in nickel atom. First start filling the electrons with lowest energy orbital and each orbital is singly occupied with one electron. And only two electrons with opposite spin can fit into an orbital. Thus, orbital diagram of nickel is as follow. Ans: 31 Add a comment Know the answer?



















0 Response to "37 construct the orbital diagram for ni."
Post a Comment