37 cs molecular orbital diagram
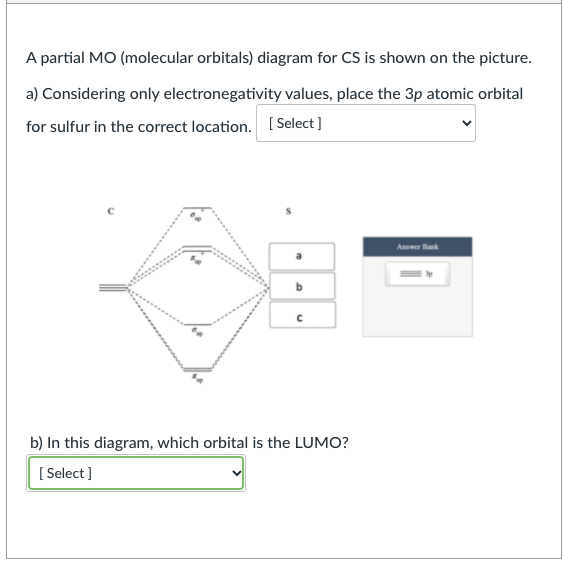
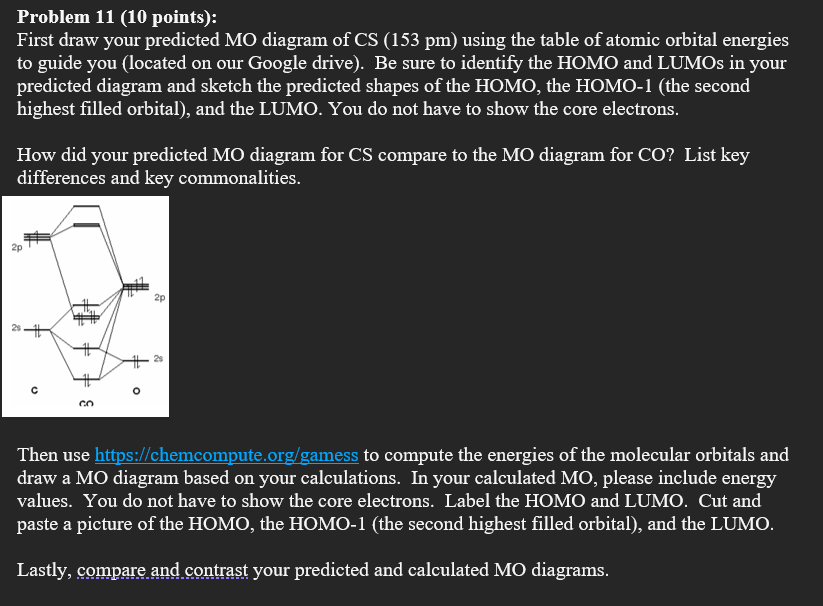
Draw the molecular orbital diagram of CS. Determine bond ... Pravin N. Answer. Draw the molecular orbital diagram of CS. Determine bond order, magnetism, spin multiplicity, and HOMO/LUMO.4 answers · Top answer: So as we know, this is the molecular vital diagram. This is the molecular vital diagram of ... What Is The Molecular Geometry Of Ccl4 Furthermore, What is the molecular geometry of CS?, The carbon atom will thus be sp hybridized. It will use one s and one p orbitals to form the hybrids, and the remaining p-orbitals to form pi bonds with the two sulfur atoms.The molecular geometry will thus be linear, the basic AX2 model.
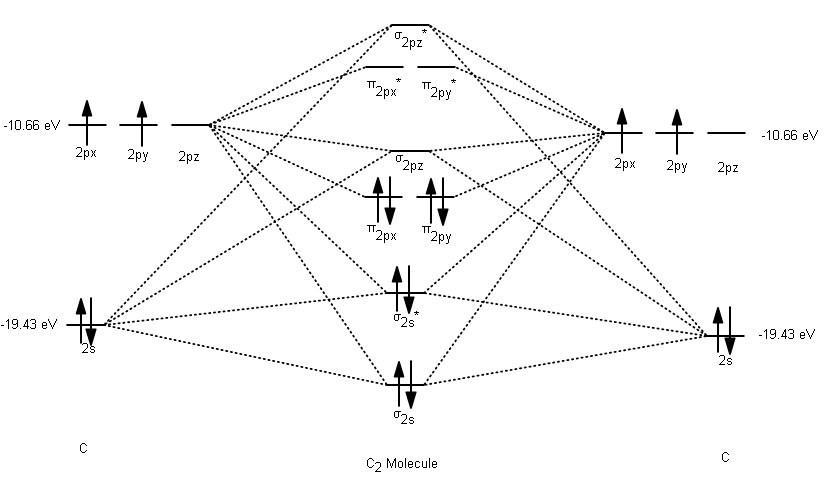
What is bond length of C2? - All Famous Faqs Hint: Recall the molecular orbital theory (MOT) and write the electronic configuration of ${C_2}$ molecule according to MOT. You will find that the ${C_2}$ molecule has two sets of paired orbitals in the degenerate pi-bonding orbitals and bond order comes out to be 2. How does the bond length vary in C2 C2 C2 2?

Cs molecular orbital diagram
Electroluminescent materials toward near ultraviolet ... Near ultraviolet (NUV) light-emitting materials and devices are significant due to unique applications in anti-counterfeit, manufacturing industries, and hygienic treatments. However, the development of high-efficiency NUV electroluminescent devices encounters great challenges and is far behind their RGB emi First-Principles Calculations for Adsorption of HF, COF2 ... HF, CS 2, and COF 2 are three important decomposition components of the SF 6 gas insulation medium. In this paper, the gas sensitivity of Pt doped on (8, 0) single-walled carbon nanotube (SWCNT) to HF, CS 2, and COF 2 is investigated based on density functional theory. The binding energy, charge transfer, density of states, and frontier molecular orbital theory are discussed. Tuning the electronic states and superconductivity in ... Alkali fullerides (A 3 C 60, A = K, Rb, Cs) have been intensively studied since the first discovery of superconductivity in K 3 C 60 in 1991 [].They exhibit a maximum superconducting transition temperature T c of ∼ 40 K, which is the highest among the molecular superconductors and only gets surpassed by cuprates and iron-based superconductors [1,2,3,4,5,6].
Cs molecular orbital diagram. › IDM_Events_NoticesInstitute Of Infectious Disease and Molecular Medicine For information on South Africa's response to COVID-19 please visit the COVID-19 Corona Virus South African Resource Portal. Molecular Orbital Theory - GeeksforGeeks Molecular orbitals provide a great model for demonstrating molecule bonding via molecular orbital theory. Types of Molecular Orbitals. According to molecular orbital theory, some types of molecular orbitals are formed by the linear combination of atomic orbitals. These orbitals are described in more detail below. Chemical Bonding Molecular Structure - Practically Study ... Bond order = 1. Nature = diamagnetic. Note : The comparison of Li - Li and H - H bonds reveals that the sigma bond in Li 2 molecule is weaker and much longer than σ bond in H 2 molecule, because 2s orbital of lithium is bigger in size than the 1s orbital of hydrogen. viii) Beryllium molecule (Be2) : Be - 1s 2, 2s 2. C2 Lewis Structure CID 160435 Methanediimine Date s. According to the molecular orbital diagram of the C22 ion you its a stable ion because it has a bond order of 1 that means its a stable substance. Electrons are shown as dots or for bonding electrons as a line between the two atoms. Because C 2 H 2 molecule is a simple molecule those all steps may not be used.
How Many Double Bonds Does CS2 Have? 2021 Practical Guide Hybridization is the fusion of atomic orbitals to create newly hybridized orbitals, which can influence bonding properties and geometry. The CS2 molecule has "sp" hybridization. From a theoretical standpoint, "sp" hybridization is formed if an "s" orbital overlaps with a "p" orbital. List in a table the Lewis structure, molecular shape,bond ... List in a table the Lewis structure, molecular shape,bond angle, and hybrid orbitals for molecules of CS, List in a table the Lewis structure, molecular shape, CH, O, H, Se, CCI,F2, and NCIz. Categories Uncategorized. Leave a Reply Cancel reply. Your email address will not be published. Topology of the Electron Density and of Its Laplacian from ... 2. Computational Details. Calculations on the molecular fragment [UO 2 Cl 4] 2 − and extended periodic crystal Cs 2 UO 2 Cl 4 are performed with the Crystal program by use of the global hybrid B3LYP exchange-correlation functional [] of the density functional theory (DFT).In the periodic calculations, reciprocal space is sampled on a regular 6×6×6 Monkhorst-Pack grid, corresponding to 68 k ... Chem 344 - Homework 9 – due Friday, Apr. 11, 2014, 2 PM Apr 11, 2014 — The energies of the MOs are (left to right). –63.9, –7.92, and +2.14 eV. Make a molecular orbital diagram for this molecule, associate the MOs ...13 pages
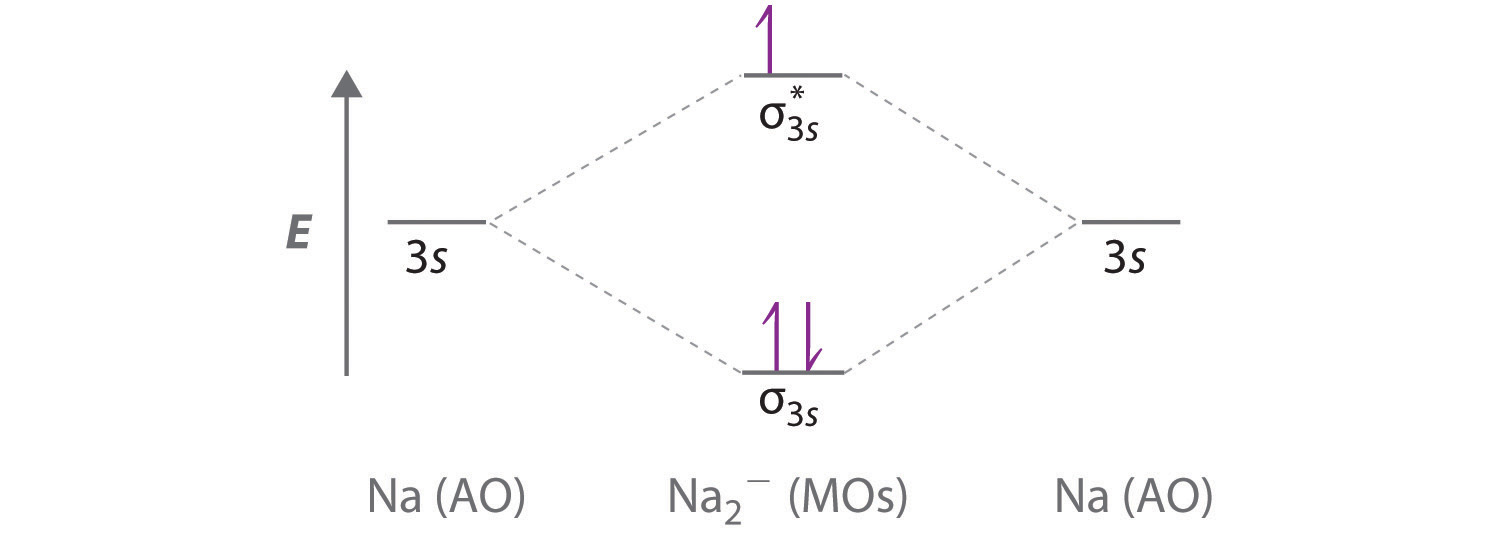
What Is The Hybridization Of The Carbon Atom In Cs2? 1 Answer. As the hybridization of CS2 is sp hybridization, the Carbon atom is in center bonding with two sulfur atoms forms the bond angle of 180 degrees, making the molecular geometry of CS2 molecule linear. The general formula for linear geometry is AX2, and thus CS2 shows linear geometry. Molecular orbital diagrams | My Assignment Tutor ... CHE 450 HW#5 Due Date: Mon. Nov. 13 Fall 2006Reading Assignments: Miessler and Tarr Inorganic Chemistry, 3rd Ed.Chapters 5, 9, & 10Volatron and Burdett (available at library reserve desk)Molecular orbital diagrams, fragment orbitals, andcorrelation diagrams(1) Generate the molecular orbital diagrams for (a) CS, (b) H3+ (linear case), and (c) H3+ (triangular case). Positron-induced scattering of acetone from 0.1 eV to 5 ... The optimized molecular wavefunction of the target was obtained from the multi-center expansion of the Gaussian-type orbitals within a Hartree-Fock self consistent field scheme. Two different models were used to account for the long-range effects arising due to the polar nature of the target. Both the models gave overlapping 'correction' CS. 4.9: Molecular Orbitals - Chemistry LibreTexts A molecular orbital diagram that can be applied to any homonuclear diatomic molecule with two identical alkali metal atoms (Li 2 and Cs 2, for example) is shown in part (a) in Figure 4.9.4, where M represents the metal atom.
Photoionization from the Xe 4d orbitals of XeF 2 (Journal ... The Journal of Chemical Physics, Vol. 111, Issue 12. DOI: 10.1063/1.479794. PCI effects and the gradual formation of Rydberg series due to photoelectron recapture, in the Auger satellite lines upon Xe 4d −1 5/2 photoionization. journal, April 2015. Kosugi, Satoshi; Iizawa, Masatomi; Kawarai, Yu.
Modern Quantum Theory - BrainMass Molecular Orbital: Example Questions. Construct a molecular orbital diagram for each geometry Which of the following are true: 1. The positively charged carbon atom contributes four valence electrons to the molecular orbitals of the methyl cation. 2. The lowest unoccupied orbital for the planar methyl cation is an sp2 hybrid orbital. 3.
NCERT Exemplar Class 11 Chemistry Unit 4 ... - AglaSem Schools (iv) CS 2; CO is isoelectronic with (i) NO + (ii) N 2 (iii) SnCl 2 ... Molecular orbitals are formed by the overlap of atomic orbitals. Two atomic orbitals combine to form two molecular orbitals called bonding molecular orbital (BMO) and anti bonding molecular orbital (ABMO). Energy of anti bonding orbital is raised above the parent atomic ...
Modifying Optoelectronic Properties of Molecular Halide ... The monomeric structure of Cs 4 PbBr 6 has an isolated [PbBr 6] 4- octahedra in the center surrounded by four Cs + cations. The determined Cs 4 PbBr 6 structure in the gas phase agrees with the reported values generated under the periodic boundary condition in the literature [ 18 ].
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ...
Valence Bond Theory - GeeksforGeeks Chemical bonds are formed by the overlapping of atomic orbitals, with electrons localised in the bond region. The valence bond theory also explains the electronic structure of molecules formed by the overlapping of atomic orbitals. It also emphasizes the fact that the nucleus of one atom in a molecule is drawn to the electrons of the other atoms.
Orbitals And Molecular Representation molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals ... The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the Because the p orbitals are all of equal energy, each of them can hold one electron before Page 1/4. Bookmark File PDF Orbitals And ...
Pentavalent Phosphorus Formation Mechanism - ScienceDirect 3-2-1: Molecular orbital diagram The analysis of the OM correlation diagram ( Figure 5 ) indicates for TS1 that the bond P-H2, which is practically formed, is stabilized because it has an energy close to its eigen-values in the product one, whereas the bond P-O is the HOMO, indicates that it is not yet formed.
Symmetry-Breaking Charge Separation in Molecular ... An effective electronic coupling (V) between a locally excited (LE) state and SB-CS state (wave function overlap between frontier molecular orbitals) is necessary for efficient charge separation to compete and win against the other decay processes such as fluorescence and non-fluorescence decay to the ground state. As a result, the approach of ...
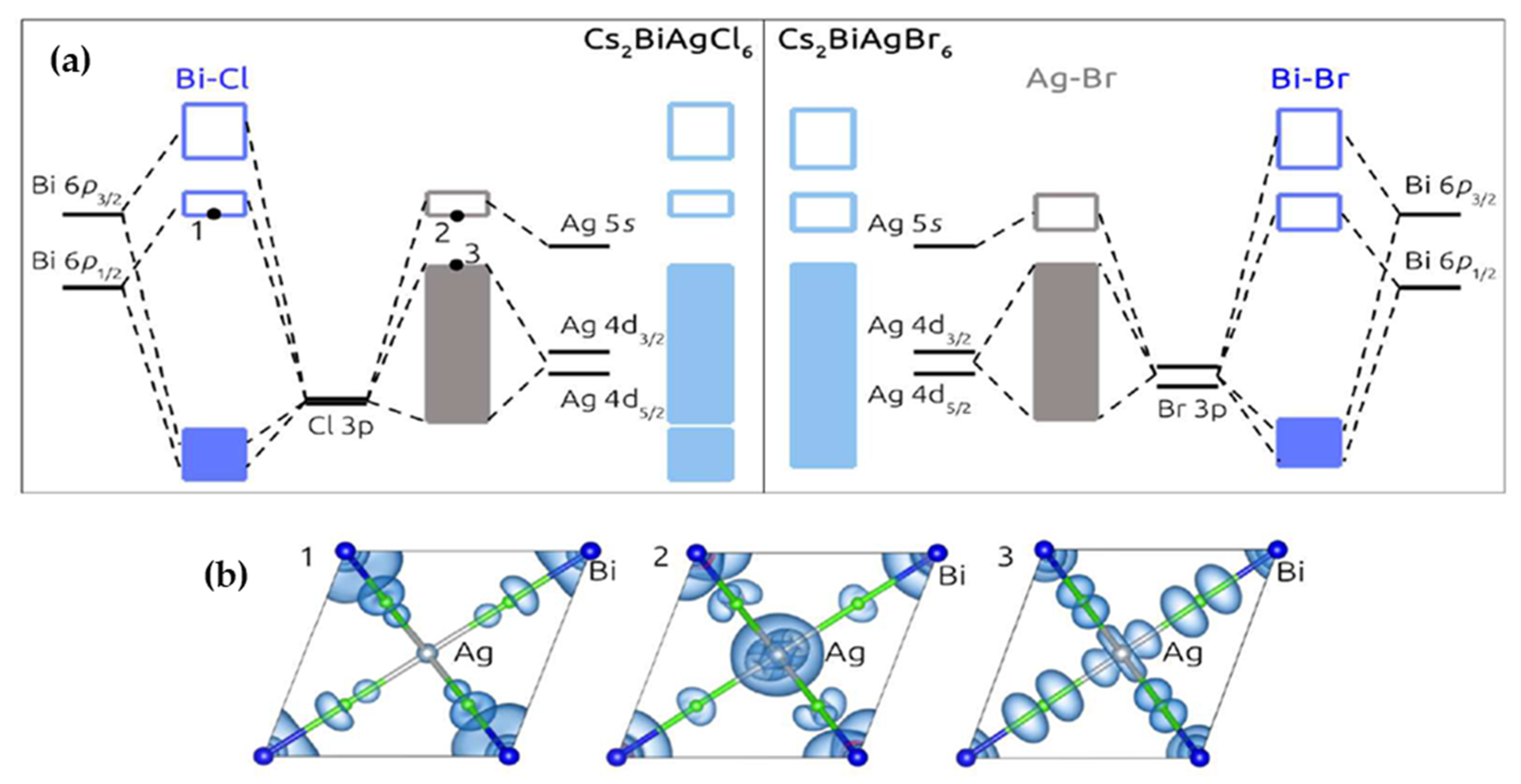
A Review on Cs-Based Pb-Free Double Halide Perovskites ... (a) Comparison between the molecular orbitals diagram of Cs 2 AgBrCl 6 and Cs 2 AgBrBr 6 and; (b) Square modulus of the wave functions for states marked from 1 to 3 on the molecular orbital diagram of Cs 2 BiAgCl 6. 1 represents the Bi-6p 1/2 /halide-p antibonding orbitals at the bottom of the conduction band (at Γpoint).
CHAPTER 5: MOLECULAR ORBITALS molecular orbitals in the diagram ... orbitals exhibit Cs symmetry. ... OF– has 14 valence electrons, four in the π2p* orbitals (see the diagram in the ...29 pages
› doi › fullA human kinase yeast array for the identification of kinases ... Feb 28, 2022 · For the growth measurement, a 96-well MTP was incubated for 1 h at 30°C with orbital shaking, the yeast was then diluted with selective media (SD5, 500 µM CuSO 4) with or without 3.2% (v/v) DMSO to a starting OD 595 of 0.10-0.15. Then yeast growth was recorded in the MTP for 24 h by taking the OD every 10 min and shaking every hour for 5 min.
Molecules | Free Full-Text | Photoionization Observables ... The Dyson orbitals are coupled to an accurate description of the electronic continuum obtained with a multicentric B-spline basis at the DFT and TD-DFT levels. Two prototype diatomic molecules, i.e., CS and SiS, have been chosen due to their smallness, which hides important correlation effects.
Question 11 Use Molecular Orbital Theory to predict the ... Question 11 Use Molecular Orbital Theory to predict the following properties of. Question 11 use molecular orbital theory to predict. School Brock University; Course Title CHEM 1F92; Type. Notes. Uploaded By eamoff. Pages 22 This preview shows page 16 - 22 out of 22 pages. ...
Orientations and water dynamics of photoinduced secondary ... The objects of the present study are 1) clarifying molecular geometries of the secondary CS state, i.e. position of oxidized tryptophan and conformation of the reduced FAD in the secondary radical ...
Initiating Electron Transfer in Doubly Curved Nanographene ... The Kohn-Sham molecular orbitals representing the LE and CS states are shown in Figures S9-S11, SI. A COSMO-like model was applied to estimate the influence of a polar environment on the electronic excitations. 22 Benzonitrile (BZN) was taken as the solvent.
Tuning the electronic states and superconductivity in ... Alkali fullerides (A 3 C 60, A = K, Rb, Cs) have been intensively studied since the first discovery of superconductivity in K 3 C 60 in 1991 [].They exhibit a maximum superconducting transition temperature T c of ∼ 40 K, which is the highest among the molecular superconductors and only gets surpassed by cuprates and iron-based superconductors [1,2,3,4,5,6].
First-Principles Calculations for Adsorption of HF, COF2 ... HF, CS 2, and COF 2 are three important decomposition components of the SF 6 gas insulation medium. In this paper, the gas sensitivity of Pt doped on (8, 0) single-walled carbon nanotube (SWCNT) to HF, CS 2, and COF 2 is investigated based on density functional theory. The binding energy, charge transfer, density of states, and frontier molecular orbital theory are discussed.
Electroluminescent materials toward near ultraviolet ... Near ultraviolet (NUV) light-emitting materials and devices are significant due to unique applications in anti-counterfeit, manufacturing industries, and hygienic treatments. However, the development of high-efficiency NUV electroluminescent devices encounters great challenges and is far behind their RGB emi


![MO diagram of T d [CsO 4 ] + , as interaction between a Cs + ...](https://www.researchgate.net/publication/317849955/figure/fig2/AS:614161100836879@1523438825309/MO-diagram-of-T-d-CsO-4-as-interaction-between-a-Cs-ion-and-an-O-4-fragment_Q320.jpg)
![Molecular orbital interaction diagram for Re[Cl(CO2)] of Cs ...](https://www.researchgate.net/publication/342215688/figure/fig5/AS:960037639970826@1605902218521/Molecular-orbital-interaction-diagram-for-ReClCO2-of-Cs-symmetry-The-populations-of.png)


![Conceptual MO-diagram of [Co(II/III)(L1) 3 ] 2+/3+ in high ...](https://www.researchgate.net/profile/Chunzhen-Yang/publication/322841939/figure/fig1/AS:589124784390144@1517469702597/Conceptual-MO-diagram-of-CoII-IIIL1-3-2-3-in-high-spin-and-low-spin-states-For_Q640.jpg)










0 Response to "37 cs molecular orbital diagram"
Post a Comment