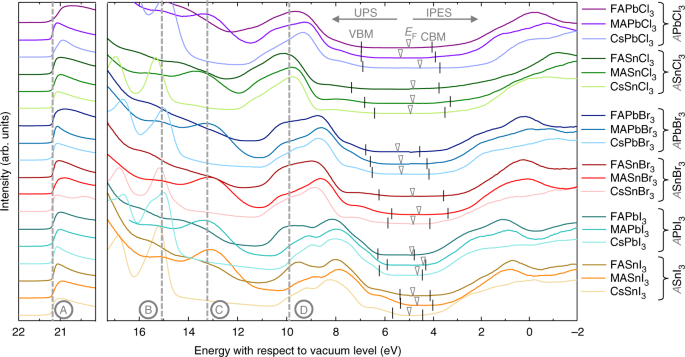
37 the figure is an energy-level diagram for a simple atom. (figure 1)
The figure is an energy-level diagram for a simple atom. For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale. Energy Level Diagram - Different Energy Shells Around the ... Energy Level Diagram What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the "K shell" followed by the "L shell" then the "M shell" and so on away from the nucleus.
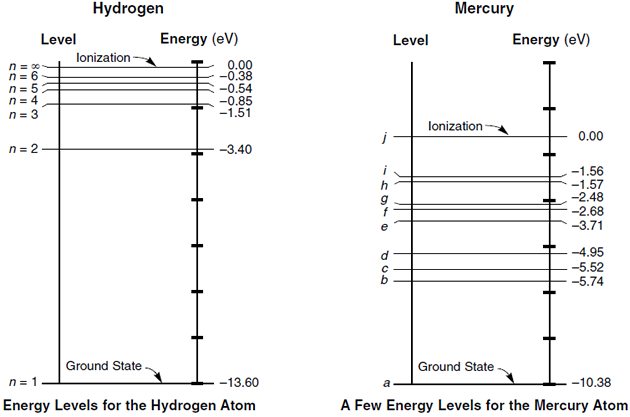
Energy Level Diagram For Hydrogen - Mini Physics The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.

The figure is an energy-level diagram for a simple atom. (figure 1)
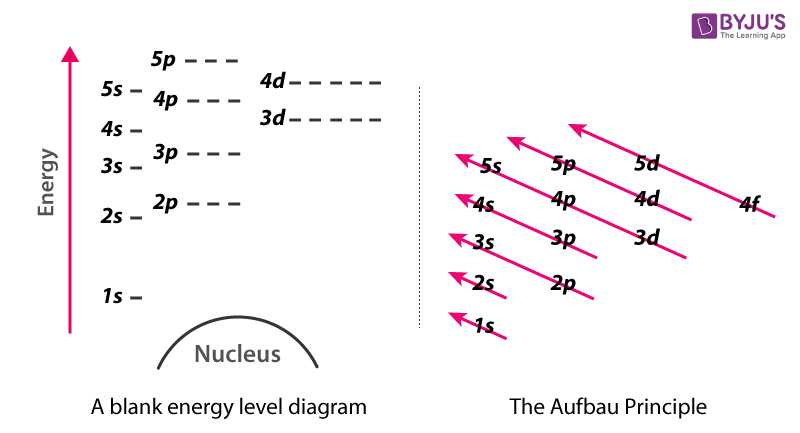
How do you calculate the energy of an electron in the ... You can calculate the ground state energy using The Bohr Model A simple expression for the energy of an electron in the hydrogen atom is: E=-(13.6)/(n^2) where the energy is in electron volts n is the principle quantum number. This gives rise to the familiar electron energy level diagram where they converge and coalesce. So for an electron in n=1: E=-13.6"eV" To convert to joules you can x ... PDF GCSE Grade (d)€€€€€Figure 3 shows the reaction between ethene and chlorine and is similar to the reaction between ethene and bromine. Figure 3 € "The more energy levels (shells) of electrons an atom has, the weaker the covalent bonds that it forms." Use the above statement to predict and explain how the overall energy change for the Energy level diagram of mercury atom. | Download ... As the name implies, Hg is the main component of low-pressure Hg lamps. Figure 1, taken from Baeva and Reiter (2003) [5], shows a diagram of the various energy levels of a Hg atom and possible...
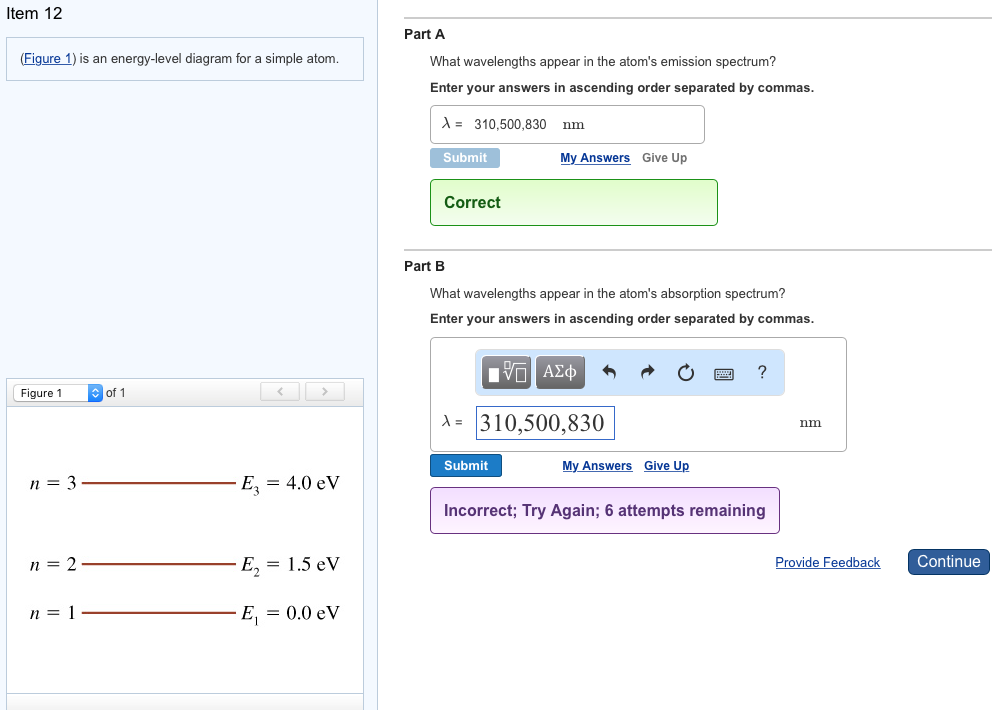
The figure is an energy-level diagram for a simple atom. (figure 1). (Figure 1) is an energy-level diagram for a simple atom ... Figure Ex38.22 is an energy-level diagram for a simple atom, inwhich E1 = 0.0 eV, E2 =1.2 eV, and E3 =3.3eV. Energy level diagrams and the hydrogen atom Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. Solved The figure is an energy-level diagram for a simple ... The figure is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? #1: From wavelength 3 to 1. #2: From wavelength 3 to 2. #3: From wavelength 2 to 1. What wavelengths appear in the atom's absorption spectrum? The following image is an energy level diagram for a simple ... The following image is an energy level diagram for a simple atom, where E1 = 0.00 eV, E2 = 1.57 eV, and E3 = 4.10 eV. i) What wavelengths appear in the atom's ...1 answer · Top answer: Given data: • The energy of the first level is E1=0.00eVE1=0.00eV • The energy of the second level is E2=1.57eVE2=1.57eV • ...
(Figure 1) is an energy-level diagram for a simple atom. What ... (Figure 1) is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? Enter your answers in ascending order ...2 answers · 3 votes: Emission and absorption spectrum has same frequency (or wave length) Part A Emission spectrum ... Carbon energy level diagram - Big Chemical Encyclopedia A part of the energy-level diagram for carbon monoxide is shown in Figure VII-4. The energy levels have been obtained by analysis of the observed frequencies of the lines in the emission and absorption spectra of the molecule. It has a node between each bonded pair of carbons and is antibonding. Figure 1.18 shows these tt MO s in an energy-level diagram. . PDF ANSWER. Series #2 - LSU Refer to Figure 1 when answering the first 7 questions of this exam. 1. Which series of electron transitions in the energy-level diagram for Hydrogen produce the lines shown in the absorption-line spectrum of Hydrogen? ANSWER. Series #2 2. Which series of electron transitions in the energy-level diagram produce the "Balmer" An energy-level diagram for a hypothetical atom is shown ... An energy-level diagram for a hypothetical atom is shown above. a. Determine the frequency of the lowest energy photon that could ionize the atom, initially in its ground state. b. Assume the atom has been excited to the state at -1.0 electron volt. i. Determine the wavelength of the photon for each possible spontaneous transition. ii.
Bohr's Theory of the Hydrogen Atom | Physics II Figure 5. An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons, showing a transition between two orbits having energies E 4 and E 2. Energy Level and Transition of Electrons | Brilliant Math ... Imgur. The energy of the electron of a monoelectronic atom depends only on which shell the electron orbits in. The energy level of the electron of a hydrogen atom is given by the following formula, where. n. n n denotes the principal quantum number: E n = − 1312 n 2 kJ/mol. E_n=-\frac {1312} {n^2}\text { kJ/mol}. E n. 22.1 The Structure of the Atom - Physics | OpenStax Figure 22.10 An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons, showing a transition between two orbits having energies E 4 E 4 and E 2 E 2. The energy transition results in a Balmer series line in an ... PDF AP PHYSICS 2011 SCORING GUIDELINES - College Board Note: Figure not to scale For a correct energy-level diagram with horizontal lines and -6 eV at the bottom 1 point Note: The energy-level diagram does not need to be drawn to scale in order to earn this point. For correct labeling of energy levels and quantum numbers (e.g., n = 1 correlated with
PDF Energy Changes Q1. - Weebly (d) Figure 3 shows the reaction between ethene and chlorine and is similar to the reaction between ethene and bromine. Figure 3 "The more energy levels (shells) of electrons an atom has, the weaker the covalent bonds that it forms." Use the above statement to predict and explain how the overall energy change for
Answered: values, and get the results.) 1. Figure… | bartleby Transcribed Image Text: values, and get the results.) 1. Figure on the right is an energy-level diagram for a simple atom. (a) What wavelengths, in nm, appear in the atom's emission spectrum? (b) What wavelengths, in nm, appear in the atom's absorption spectrum? n = 3 E, = 4.00 eV n = 2 E, = 1.50 eV n = 1 E, = 0.00 eV. Expert Solution.
Solved Part A (Figure 1) is an energy-level diagram for a Transcribed image text: Part A (Figure 1) is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum?1 answer · Top answer: The atom can have transition from 4.0ev to 1.5 , 0 and 1.5 to 0 the em...
Atomic Energy Levels (video) - Khan Academy We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV.
Draw a neat labelled energy level diagram of the Hydrogen ... The energy gap between successive energy levels in a hydrogen atom: Answer the following questions. (i) Draw the energy level diagram showing the emission of b-particles followed by y-rays by a 2 7 6 0 C o nucleus. (ii) Plot of distribution of KE of b-particles as drawn in section i. A rectangular corral of widths L x = L and L y = 2 L contains ...
Solved (Figure 1) is an energy-level diagram for a simple ... Question: (Figure 1) is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? Enter your answers in ascending order separated by commas. What wavelengths appear in the atom's absorption spectrum? Enter your answers in ascending order separated by commas.
PDF This question is about the reaction of ethene and bromine ... Figure 3 € "The more energy levels (shells) of electrons an atom has, the weaker the covalent bonds that it forms." Use the above statement to predict and explain how the overall energy change for the reaction of ethene with chlorine will differ from the overall energy change for the reaction of ethene with bromine.
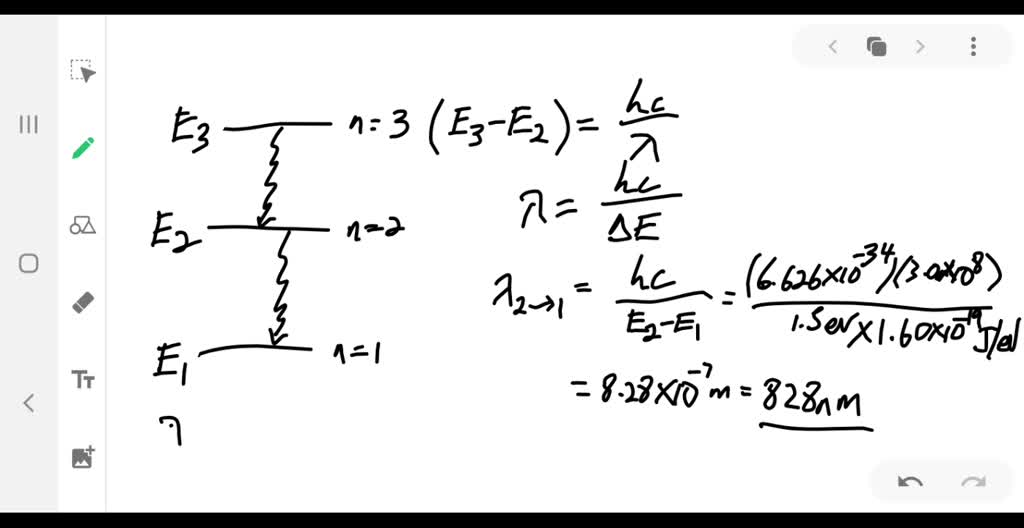
figure ex3920 is an energy level diagram for a simple atom what wavelengths appear in the atoms a em
Energy Level of an Atom - Energy State and ... - VEDANTU The figure shows the energy levels of an atom. The first four energy levels are shown here. The first energy level is also called level 'K'. The second level is called level L, third energy level as M, and so on. The electrons from energy level K contains the least energy whereas the levels that are far from the nucleus contains more energy
The diagram above shows part of an energy-level diagram ... The diagram above shows part of an energy-level diagram for a certain atom. The wavelength of the radiation associated with transition A is 600 nm (1 nm = 1 x 10 -9 m) and that associated with transition B is 300 nm. a. Determine the energy of a photon associated with transition A. b. Determine the λ of the radiation associated with transition C
PhysicsLAB: Energy-Level Diagrams Using the Bohr Model, the energy levels (in electron volts, eV) are calculated with the formula: En = -13.6 (Z2/n2) eV where Z is the atomic number and n is the energy level. The ground state is represented by n = 1, first excited state by n = 2, second excited state by n = 3, etc. eV
Schematic diagram of the energy levels of aluminium atom ... Figure 5 shows the energy levels and the resonance lines of Al atom [18]. These two lines have a common upper level 2 S 1 / 2 with an energy of 3.143 eV. These two lines have a common upper level ...
PDF 4. Energy Levels - MIT OpenCourseWare Energy Levels 4.1 Bound problems 4.1.1 . Energy in Square infinite well (particle in a box) 4.1.2 . Finite square well 4.2. Quantum Mechanics in 3D: Angular momentum 4.2.1 . Schrodinger equation in spherical coordinates 4.2.2 . Angular momentum operator 4.2.3 . Spin angular momentum 4.2.4 . Addition of angular momentum 4.3
OneClass: The figure is an energy-level diagram for a ... For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale.
Energy level diagram of mercury atom. | Download ... As the name implies, Hg is the main component of low-pressure Hg lamps. Figure 1, taken from Baeva and Reiter (2003) [5], shows a diagram of the various energy levels of a Hg atom and possible...
PDF GCSE Grade (d)€€€€€Figure 3 shows the reaction between ethene and chlorine and is similar to the reaction between ethene and bromine. Figure 3 € "The more energy levels (shells) of electrons an atom has, the weaker the covalent bonds that it forms." Use the above statement to predict and explain how the overall energy change for the
How do you calculate the energy of an electron in the ... You can calculate the ground state energy using The Bohr Model A simple expression for the energy of an electron in the hydrogen atom is: E=-(13.6)/(n^2) where the energy is in electron volts n is the principle quantum number. This gives rise to the familiar electron energy level diagram where they converge and coalesce. So for an electron in n=1: E=-13.6"eV" To convert to joules you can x ...




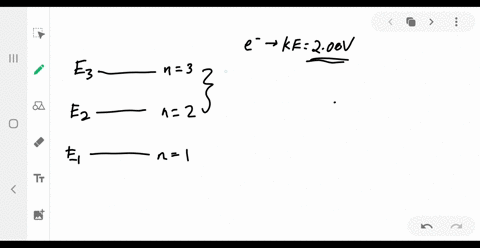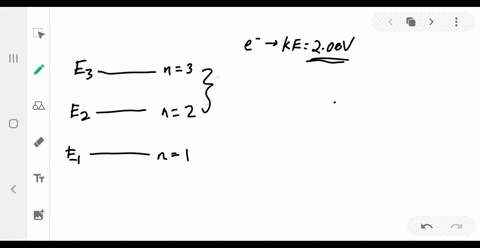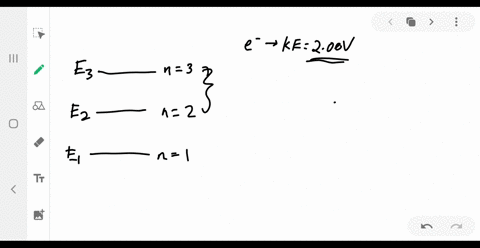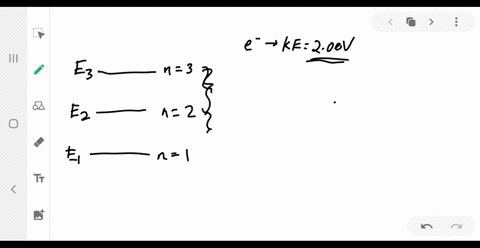











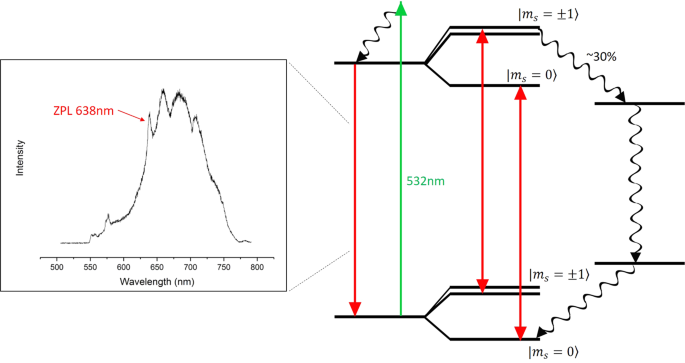




0 Response to "37 the figure is an energy-level diagram for a simple atom. (figure 1)"
Post a Comment