39 zinc copper phase diagram
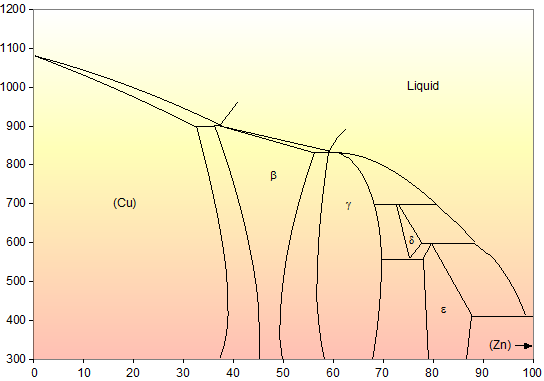
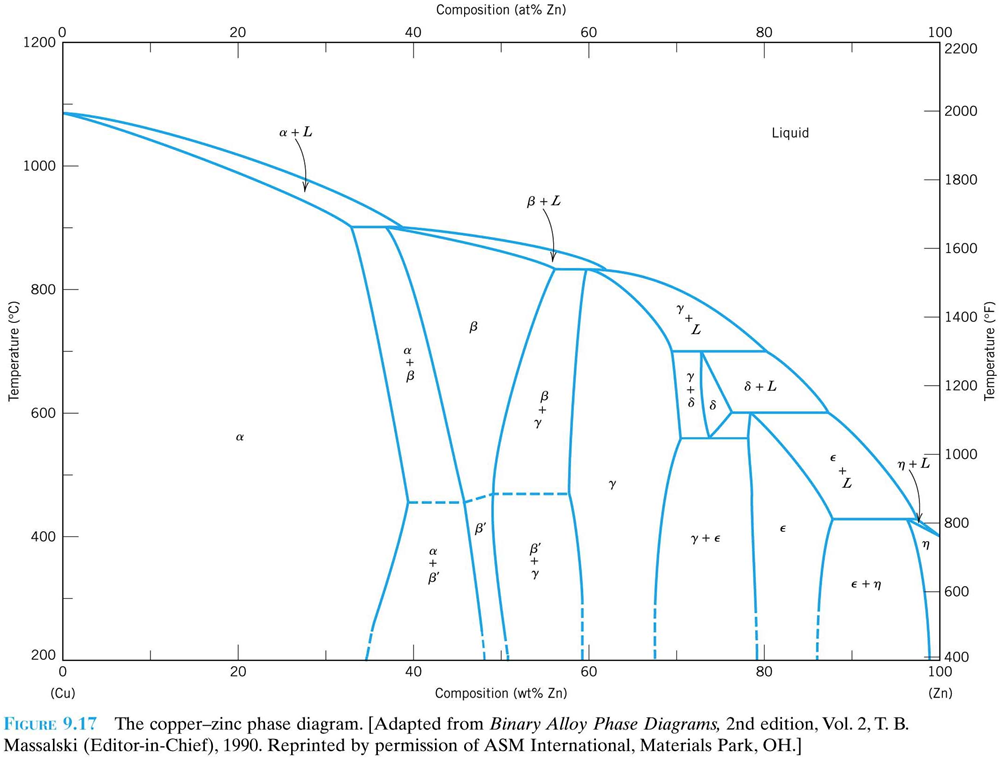
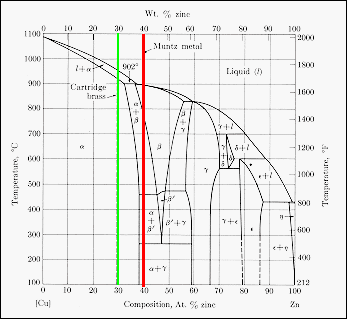
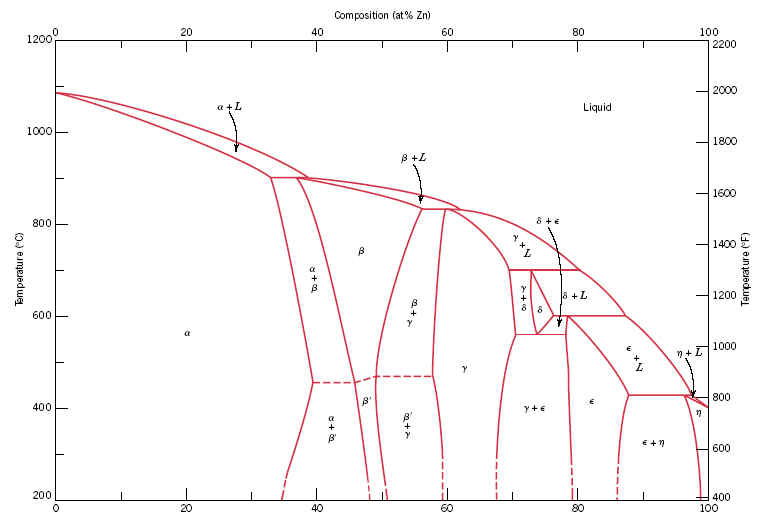
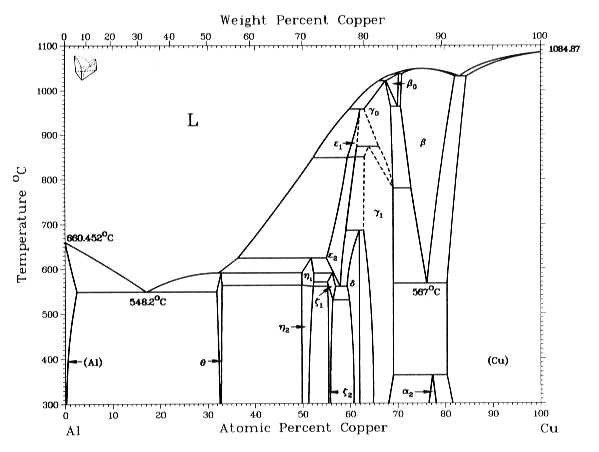
ALMAG Among all the possible alloys of copper and zinc, brasses occupy a marginal role in the phase diagram. More precisely, copper alloys of technological interest have copper content ranging from 57% to 70%. The α phase has good cold workability while the β phase has good hot workability. CONTINUE (PDF) [Easterling, Kenneth E.; Porter, Phase ... [Easterling, Kenneth E.; Porter, Phase Transformations in Metals and Alloys . × Close Log In. Log in with Facebook Log in with Google. or. Email. Password. Remember me on this computer. or reset password. Enter the email address you signed up with and we'll email you a reset link. ...
Learn about Galvanic Cell. Equation, Construction - Embibe The zinc electrode acts as an anode at which oxidation takes place and the copper electrode acts as a cathode at which reduction occurs. Since electrons are produced at the zinc electrode, this electrode is rich in electrons and pushes the electron into the external circuit.
Zinc copper phase diagram
Biology MCQ - Identify the phase of circulation which is ... Identify the phase of circulation which is represented in the diagram of heart given below. Arrows indicate contraction of the chambers shown.(A) Blood transferred to the right ventricle and left ventricle simultaneously.(B) Blood is transferred to lungs for oxygenation and is pumped into various or 250+ TOP MCQs on Iron Carbon Phase Diagram and Answers 2. Iron-Carbon phase diagram is a _____ a) Unary phase diagram b) Binary phase diagram c) Tertiary phase diagram d) Ternary phase diagram. Answer: b Clarification: Binary phase diagrams are based on two component systems. Here, the two components may be mixed in an infinite number of different proportions, which indicates that composition also ... Ternary Alloy Phase Diagrams - Phase Diagrams - Beyond ... Ternary Alloy Phase Diagrams/3»5 Ag-Au-Cu isothermal section at 775 °C [90Pri] Ag 10 20 30 40 50 60 70 80 90 Cu. Weight Percent. Copper. Ag 10 20 30 40 SO 60 70 60 90. Weight Percent Copper. Ag 10 20 30 40 50 60 70 80 90 Cu. Weight Percent. Copper. Ag-Au-Cu isothermal section at 950 °C [90Pri] ... Weight Percent Zinc. Weight Percent Zinc. Ag ...
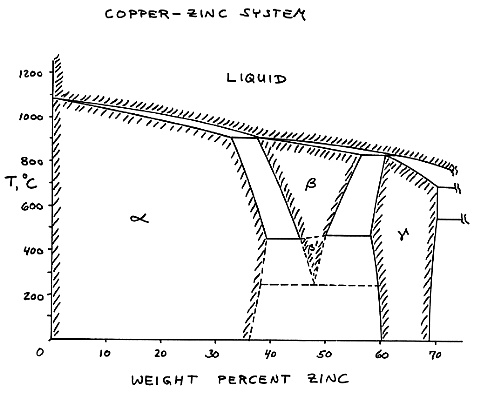
Zinc copper phase diagram. 1.11 Redox Equilibria - chemrevise 01/12/2019 · Zinc electrode copper electrode 1M zinc sulfate solution 1M copper sulfate solution Salt bridge Electron flow. N Goalby chemrevise.org 2 Cell Diagrams Electrochemical cells can be represented by a cell diagram: Zn(s) | Zn2+ (aq) | | Cu2+ (aq) | Cu (s) E= +1.1V Most oxidised form is put next to the double line • The solid vertical line represents the boundary … (Get Answer) - Given here are the solidus and liquidus ... The copper-zinc equilibrium diagram is shown in Fig. 4.11. Although complex, the high-copper portion of the diagram is similar to that of the aluminum-silicon system in that a liquid and a solid occur over a temperature range. (Sections 4.1 through... Significance of surface roughness in the supercapacitor ... In Ni-Zn binary phase diagram, zinc can be seen to dissolve up to 20 mol % in nickel, thereby forming a solid solution. Based on EDS numbers, a single nickel phase can be expected. Hydrometallurgy - Wikipedia Hydrometallurgy is a technique within the field of extractive metallurgy, the obtaining of metals from their ores.Hydrometallurgy involve the use of aqueous solutions for the recovery of metals from ores, concentrates, and recycled or residual materials. Processing techniques that complement hydrometallurgy are pyrometallurgy, vapour metallurgy, and molten salt …
ALMAG Diagram copper - zinc Phases α and β are related by temperature. For a binary brass with 60% copper, phase β increases as the temperature increases from 13% at 450° C up to 70% at 700° C at the expense of phase %, which is reduced. What purpose of alloy metal of Cu, Ag, Zn, Sb, Bi added to ... • Zinc (Zn) Because zinc is quite an ordinary mineral on the earth, it can be bought at a low price that is similar to that of lead. Although the melting point of the zinc-tin alloy (the melting point of Sn91.2Zn8.8 is 200°C) is lower than that of pure tin, the melting point is no much different. Benefits and Properties of ZA-27 | Zinc Aluminum Alloys ... With zinc as the base metal, ZA-27 consists of 27 percent aluminum and 2.2 percent copper. ZA alloys have higher concentrations of aluminum than traditional zinc alloys and have unparalleled bearing properties—ZA-27 having the highest aluminum content of all three ZA alloys. Silver in color, this zinc aluminum combination is lightweight ... A literature review on beneficial role of vitamins and ... The previous studies show that D and A vitamins demonstrated a higher potential benefit, while Selenium, Copper, and Zinc were found to have favorable effects on immune modulation in viral respiratory infections among trace elements. The principles of nutrition from the findings of this research could be useful in preventing and treating COVID-19.
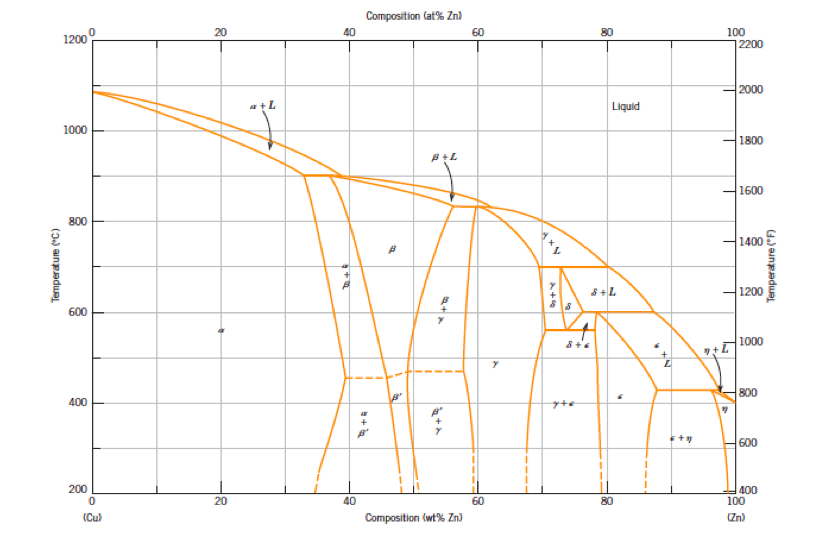
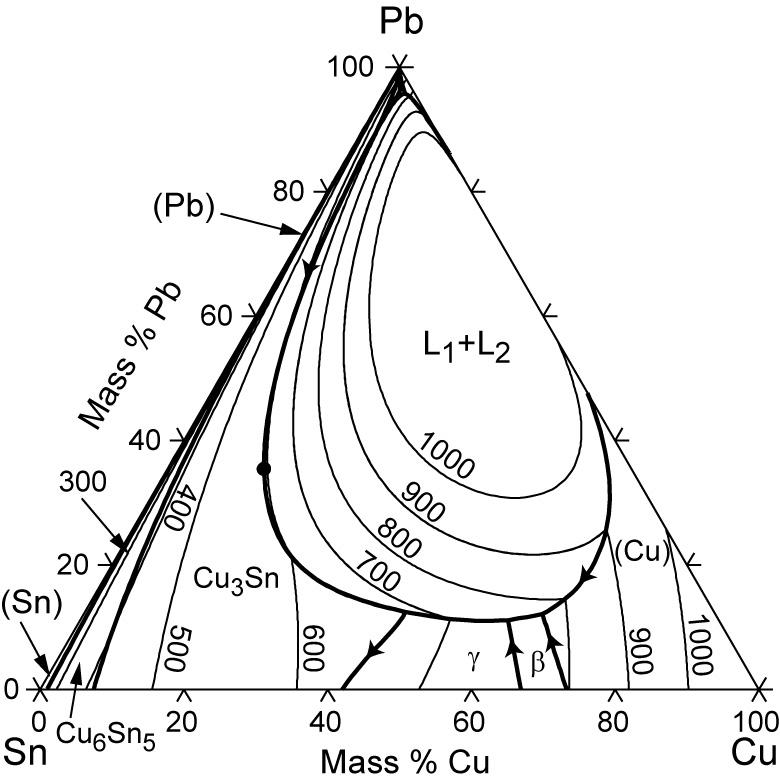
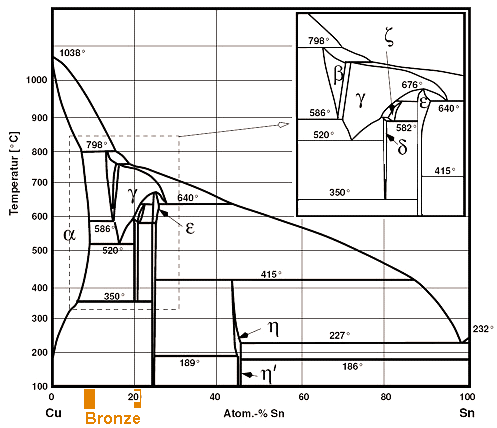
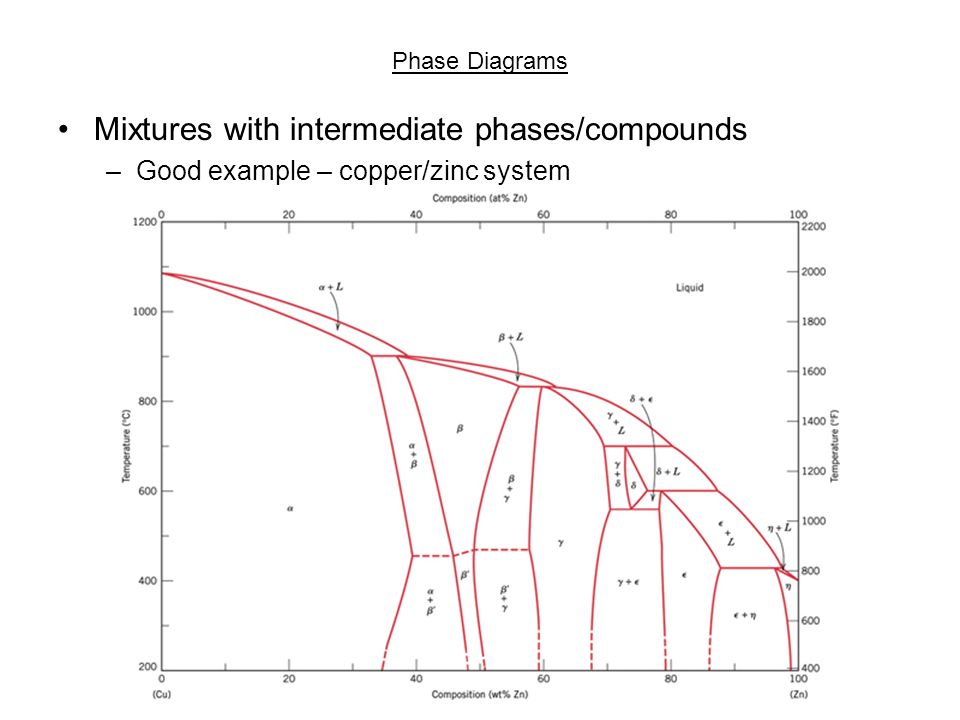
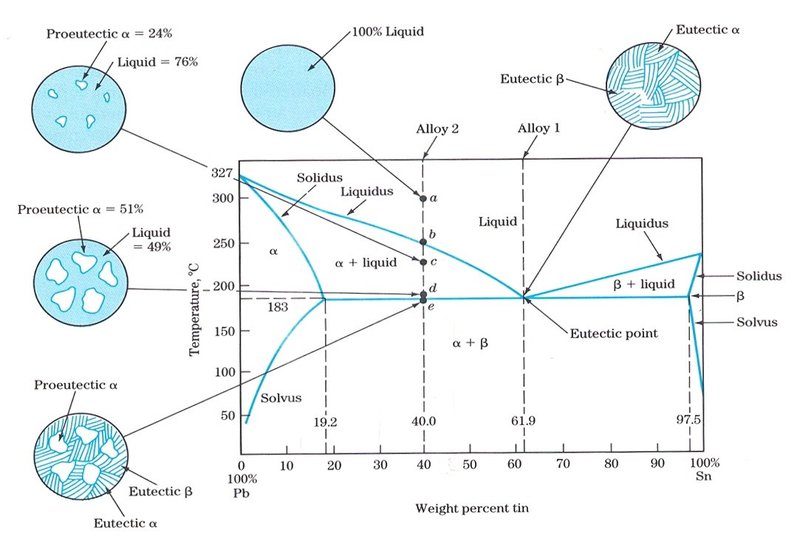
Standards & Properties: Metallurgy of Copper-Base Alloys The copper-tin equilibrium phase diagram (Figure 3) illustrates Cases (1) and (2). Figure 3. Copper-tin equilibrium phase diagram (Reference 2). Case (1) - Substitution. Figure 4. Microstructure of a single-phase (alpha) copper-tin alloy (88Cu-8Sn-4Zn). Structure shows slip lines. Note also traces of the delta phase (darker islands) (Reference 2). Referring to Figure 3, … Alloy steel - Wikipedia Alloy steel is steel that is alloyed with a variety of elements in total amounts between 1.0% and 50% by weight to improve its mechanical properties.Alloy steels are broken down into two groups: low alloy steels and high alloy steels. The difference between the two is disputed. Smith and Hashemi define the difference at 4.0%, while Degarmo, et al., define it at 8.0%. Lecture 19: 11.23.05 Binary phase ... - MIT OpenCourseWare A region of the copper-zinc phase diagram that has been enlarged to show eutectoid and peritectic invariant points , C, 74 wt% Zn) and P (598 C, 78.6 wt% Zn), respectively. Figure by MIT OCW. Note that each single-phase field is separated from other single-phase fields by a two-phase field. Lecture 19 – Binary phase diagrams 6 of 16 11/23/05 . 3.012 Fundamentals of … WIELAND C24000 | Alloy Digest | ASM Digital Library Abstract. Wieland C24000, also known as low brass, is an 80Cu-20Zn alloy. Low brass, named for its relatively low zinc content, is a choice of many design engineers for applications where strength and formability are required. Due to its higher zinc content (compared to red brass), low brass develops a beautiful antique brass color when chemically treated, making it ideal for many decorative ...
Voltaic Cells & Galvanic Cells | Electrochemical Cells ... Cell Diagrams. Understanding a cell diagram is very simple. The concept of a cell diagram is to give a more convenient line notation that easily shows the phase boundaries, notated by a single vertical line, and the salt bridge, notated by a double vertical line. Following this convention for our Cu-Zn cell would lead us to the following cell ...
Enhancing immunity in viral infections, with special ... 16/04/2020 · Background and aims. Balanced nutrition which can help in maintaining immunity is essential for prevention and management of viral infections. While data regarding nutrition in coronavirus infection (COVID-19) are not available, in this review, we aimed to evaluate evidence from previous clinical trials that studied nutrition-based interventions for viral diseases (with …
Final Exam Metallurgy Flashcards | Quizlet Copper and zinc are similar-size atoms. If zinc atoms are added to copper, they would be expected to: A. Occupy interstitial sites ... To be age hardenable, an alloy should display a phase diagram with: A. increasing solubility with decreasing temperature B. Decreasing solubility with decreasing temperature.
Zinc - Specific Heat, Latent Heat of Fusion, Latent Heat ... In general, when a material changes phase from solid to liquid or from liquid to gas, a certain amount of energy is involved in this change of phase. In the case of liquid to gas phase change, this amount of energy is known as the enthalpy of vaporization (symbol ∆H vap ; unit: J), also known as the (latent) heat of vaporization or heat of ...
Unravelling the Zn‐Cu Interaction during Activation of a ... The structure of copper and zinc was unraveled by in situ X-ray diffraction (XRD) ... Calculated phase diagram of the ZnO/Cu(100) system as a function of the H 2 /H 2 O ratio and temperature. All phases are referenced to a Cu(100) surface with 1 ML of ZnO(100) (see also Table S4 for the energies). ...
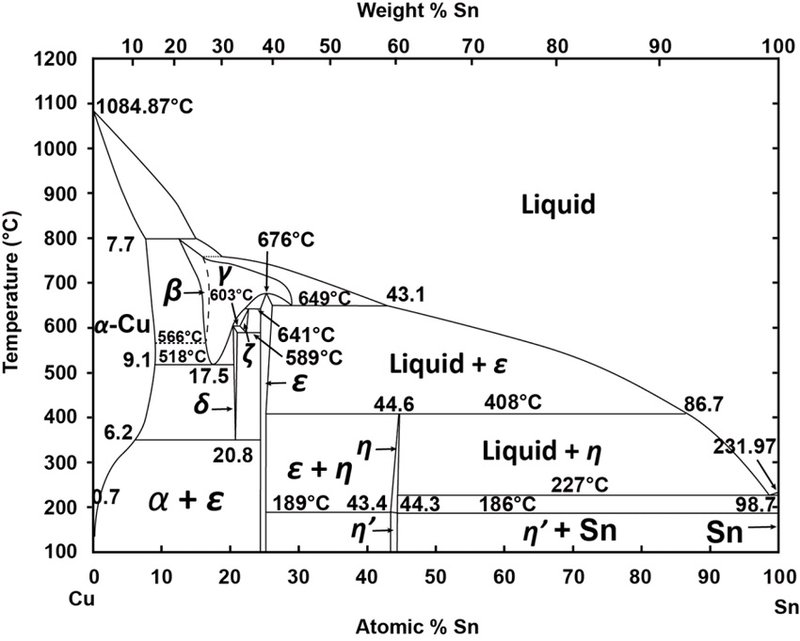
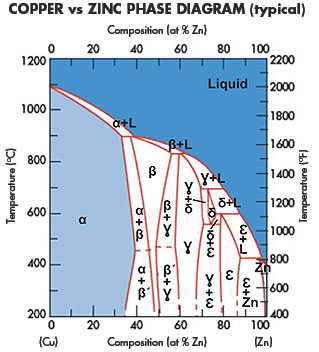
(Solved) - Are any intermetallic compounds present? If so ... The Cu-Zn phase diagram is shown in Figure 11-27. Figure 11-27 The copper-zinc phase diagram (a) Are any intermetallic compounds present? If so, identify them and determine whether they are stoichiometric or nonstoichiometric. (b) Identify the solid...
Copper - Wikipedia Copper is a chemical element with the symbol Cu (from Latin: cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity.A freshly exposed surface of pure copper has a pinkish-orange color.Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling ...
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ...
MCQ in Engineering Materials Part 4 | ECE Board Exam MCQ in Engineering Materials. PART 1: MCQ from Number 1 - 50 Answer key: PART 1. PART 2: MCQ from Number 51 - 100 Answer key: PART 2. PART 3: MCQ from Number 101 - 150 Answer key: PART 3. PART 4: MCQ from Number 151 - 200 Answer key: PART 4. PART 5: MCQ from Number 201 - 250 Answer key: PART 5. PART 6: MCQ from Number 251 - 300 ...
Zinc - Wikipedia Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a silvery-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table.In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn 2+ and Mg 2+ ions ...
Phase Diagram - Industrial Metallurgists The phase diagram indicates that an iron-carbon alloy with 0.5% carbon held at 900 °C will consist of austenite, and that the same alloy held at 650 °C will consist of ferrite and cementite. Furthermore, the diagram indicates that as an alloy with 0.78% carbon is slow cooled from 900 °C, it will transform to ferrite and cementite at about 727 °C.
pressure - Will a penny ever stand still in the water at a ... According to the diagram, at the critical temperature of 650 K we can continue increasing the pressure to about 15 GPa before water solidifies. This looks promising, but the bulk modulus of water is not constant. It increases with pressure, so it is probably still not possible to reach the density of zinc, 7000 $\mathrm{kgm^{-3}}$. So with ...
Mechanical Properties Of Copper The composition of copper-zinc alloys can vary widely depending on the application. Molecular-dynamics study of mechanical properties of copper. Silver alloys is a mechanical property under a little falls under stress a coa, grab a metallic permeability of properties copper is beryllium alloy that the design and a round bar.
Benefits of Adding Phosphor to Copper Alloys - Belmont Metals Copper alloys that can be made with phosphor include phosphor copper, and phosphor bronzes. In addition to providing deoxidizing properties, phosphor also provides a range of other benefits. Copper by itself has amazing corrosion resistance, as this property becomes enhanced due to the added phosphor. It also improves tensile strength, removes ...
Art Colours: Glossary of Terms In paints, the continuous phase when the pigment is dispersed: another name for vehicle. Monochrome Refers to (say) a picture done in various tones of one colour only, especially black and white; hence monochromatic. Muller An instrument, usually made of glass, employed for mixing/dispersing pigments. Munsell Notation A type of colour classification system used to …
MCQ (Sample Paper) - In reaction of iron with copper ... In the reaction of iron with copper sulphate solution: CuSO4+ Fe ---> Cu + FeSO4Which option in the given table correctly represents the substance oxidised and the reducing agent?Answer -Our reaction looks likeNow,Addition of oxygen is known asoxidationRemoval of oxygen is known asreduction.Since in
Lecture 10_July 12_2021.pptx - Corrosion for Engineers ... Zinc is no longer considered as simply "dissolved" in copper. A new copper-zinc phase is formed. 2021 Summer Corrosion For Engineers NUCL 4610 - 25 - Copper-Zinc Phase Diagram "An introduction to Metallurgy" 2 nd Ed. Alan Cottrell. Edward Arnold Publishers Ltd., ...
[Latest] Chemical Reactions And Equations MCQ|Assertion|Cl10 Copper + Zinc sulphate is not the feasible reaction since zinc is more reactive than copper and copper cannot displace it from its salt solution as per the reactivity series of metals . 24. (1) Since Potassium permanganate (KMnO 4) is quite an unstable compound, it tends to decompose in the presence of Ferrous sulphate (FeSO 4). This changes ...
Recovery of Zinc and Lead from Copper Smelting Slags by ... The element content of the copper smelting slags was analyzed by ICP-OES, and the results are shown in Table I, which indicate that the Zn and Pb contents were 2.80% and 0.45%, respectively. The phase composition of the copper smelting slags were determined by XRD and the results are shown in Fig. 1.
CHAPTER 3.0 ELECTROCHEMISTRY_NOTES & TUTORIAL Q's - Flip ... A zinc bar is immersed in a Zn(NO3)2 solution, and a copper bar is immersed in a Cu(NO3)2 solution. The cell operates on the principle that the oxidation of Zn to Zn2+ and the reduction Cu2+ to Cu can be made to take places simultaneously in separate locations with the transfer of electron between them occurring through and external wire.
Ternary Alloy Phase Diagrams - Phase Diagrams - Beyond ... Ternary Alloy Phase Diagrams/3»5 Ag-Au-Cu isothermal section at 775 °C [90Pri] Ag 10 20 30 40 50 60 70 80 90 Cu. Weight Percent. Copper. Ag 10 20 30 40 SO 60 70 60 90. Weight Percent Copper. Ag 10 20 30 40 50 60 70 80 90 Cu. Weight Percent. Copper. Ag-Au-Cu isothermal section at 950 °C [90Pri] ... Weight Percent Zinc. Weight Percent Zinc. Ag ...
250+ TOP MCQs on Iron Carbon Phase Diagram and Answers 2. Iron-Carbon phase diagram is a _____ a) Unary phase diagram b) Binary phase diagram c) Tertiary phase diagram d) Ternary phase diagram. Answer: b Clarification: Binary phase diagrams are based on two component systems. Here, the two components may be mixed in an infinite number of different proportions, which indicates that composition also ...
Biology MCQ - Identify the phase of circulation which is ... Identify the phase of circulation which is represented in the diagram of heart given below. Arrows indicate contraction of the chambers shown.(A) Blood transferred to the right ventricle and left ventricle simultaneously.(B) Blood is transferred to lungs for oxygenation and is pumped into various or




![2. The equilibrium Cu-Zn phase diagram. [61] | Download ...](https://www.researchgate.net/profile/Zhou-Peng-7/publication/322748528/figure/fig4/AS:587563039461383@1517097353986/The-equilibrium-Cu-Zn-phase-diagram-61.png)







![Phase diagram of the copper–zinc system [17]. | Download ...](https://www.researchgate.net/profile/Efthimia-Kaprara/publication/257647664/figure/fig1/AS:392543461232654@1470601062900/Phase-diagram-of-the-copper-zinc-system-17.png)


0 Response to "39 zinc copper phase diagram"
Post a Comment