40 orbital diagram for silicon
What is the electron orbitals of silicon? - Answers Silicon has an a atomic number of 14. Therefore it has 14 protons and 14 electrons to keep it neutral. The first 14 electrons are placed into the following orbitals: 1s2 2s2 2p6 3s2 3p2. Electron configuration of silicon (Si), orbital diagram ... Orbital Diagram, electron configuration, and the noble gas notation for a silicon (Si) atom.
Silicon (Si) - Periodic Table (Element Information & More) 2 × 4² = 32. Thus, 1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. 3rd shell can hold 18 electrons. 4th shell can hold 32 electrons. Now the atomic number of silicon (Si) is 14. Hence the silicon element has electrons arrangement 2, 8, 4.
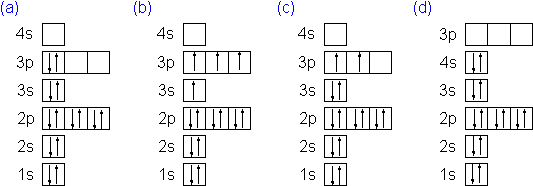
Orbital diagram for silicon
Bismuth(Bi) electron configuration and orbital diagram The 4f orbital is now full of electrons. So, the next ten electrons will enter the 5d orbital and the remaining three electrons will enter the 6p orbital. Therefore, the bismuth(Bi) electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10 6s 2 6p 3. How to write the orbital diagram for bismuth(Bi)? Silicon(Si) electron configuration and orbital diagram Orbital diagram for silicon (Si) Silicon (Si) excited state electron configuration Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of silicon is 1s2 2s2 2p6 3s2 3p2. The valency of the element is determined by electron configuration in the excited state. Silicon Electron Configuration (Si) with Orbital Diagram Silicon Orbital Diagram Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form. That is one box contains 2 electrons. And for silicon, there will be 7 box representations for 14 electrons in a pair.
Orbital diagram for silicon. What is the orbital configuration for silicon ... The 4s orbital is filled ; How many electrons does a silicon atom have? Although the silicon atom has 14 electrons their natural orbital arrangement allows only the outer four of these to be given to accepted from or shared with other atoms. Silicon is a chemical element with the symbol Si and atomic number 14. Orbital diagrams must follow 3 rules. Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration February 15, 2021 by Sneha Leave a Comment Nitrogen Electron Configuration : When we talk about school subjects, then one of the major subjects which are very important for knowledge perspective is science . Chem4Kids.com: Silicon: Orbital and Bonding Info Silicon atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many ... 7.9: Comparison Between Silicon and Carbon - Chemistry ... Figure \(\PageIndex{1}\): Molecular orbital diagram for SiF 6 2-. Electronegativity. The electronegativities of silicon and carbon are given in Table along with hydrogen. Since carbon is more electronegative than hydrogen the C-H bond is polarized towards carbon resulting in a more protic hydrogen (Figure \(\PageIndex{2}\)a). ...
What is the orbital diagram for silicon? | Study.com The Orbital Diagram for Silicon: The orbital diagram for an element shows the electron distribution of the electrons, and the correct pairing of electrons with respect to electron spin. Orbital Diagram For Arsenic - schematron.org The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! Arsenic atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Can somebody explain me what are the vacant 3d orbitals of ... silicon only has 14 electrons The reason is that Silicon only has 14 electrons. When you start filling up the orbitals, you do that from the lowest energy and in this order: "1s" -> "2s" -> "2p" -> "3s" -> "3p" -> "4s" -> "3d"... . As the levels get larger, their sublevels start to overlap. That's why 4s is at a lower energy than 3d sublevel. Each sublevel can accommodate a fixed number of ... 8.4 Molecular Orbital Theory – Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
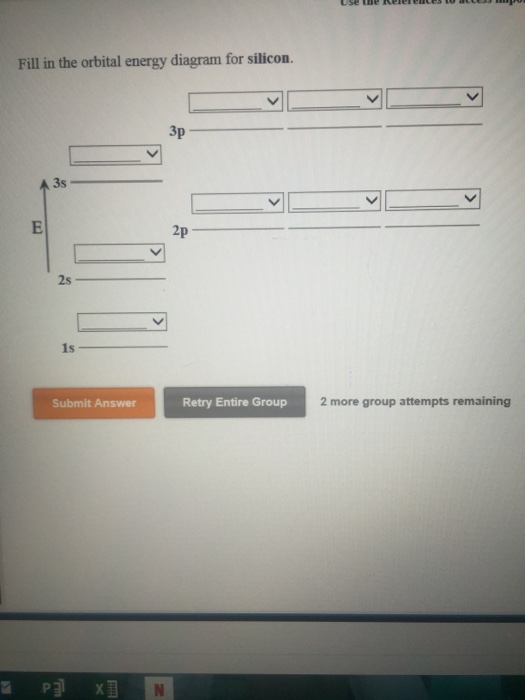
Electron Configuration for Silicon (Si) - UMD In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. Probing the orbital angular momentum of intense vortex ... 08.02.2022 · Optical vortex beams carry the well-known optical orbital angular momentum (OAM) of ℓћ per photon 1, where ℓ is usually an integer number … Orbital Energy Diagram For Silicon - schemaeasy.com The Orbital Diagram for Silicon. Silicon and Its Use the orbital diagram to find the third and eighth electrons. So, the next two electrons will enter the 4s orbital and the remaining three electrons will enter the 3d orbital. Ans: Four valence electrons. XeF2 Lewis Structure, Molecular Geometry ... - Techiescientist Apr 28, 2022 · To learn about the different properties and characteristics, it is important to have an idea about chemical bonding. In this article, we have discussed Lewis Structure, Molecular Geometry, Hybridization, and Molecular Orbital Theory-based Diagram of XeF2. Now, it’s time for you to go through this and understand chemistry a little better.
Silicon Facts (Atomic Number 14 or Si) - ThoughtCo Silicon is the eighth most abundant element in the universe. Silicon crystals for electronics must have a purity of one billion atoms for every non-silicon atom (99.9999999% pure). The most common form of silicon in the Earth's crust is silicon dioxide in the form of sand or quartz. Silicon, like water, expands as it changes from liquid to solid.
Solar cell - Wikipedia Diagram of the possible components of a photovoltaic system. Assemblies of solar cells are used to make solar modules that generate electrical power from sunlight, as distinguished from a "solar thermal module" or "solar hot water panel". A solar array generates solar power using solar energy. Cells, modules, panels and systems. Multiple solar cells in an integrated group, all …
SiF4 Lewis Structure, Molecular Geometry, Hybridization ... Step 1: Find out the total number of valence electrons in silicon tetrafluoride. Silicon tetrafluoride contains one silicon atom and four fluorine atoms. The silicon and fluorine atoms belong to group 14, and group 17, respectively. Hence, the silicon atom has four valence electrons whereas the fluorine atom has seven valence electrons.
Electron configuration for Molybdenum (element 42 ... Orbital diagram. Molybdenum electron configuration ← Electronic configurations of elements . Mo (Molybdenum) is an element with position number 42 in the periodic table. Located in the V period. Melting point: 2617 ℃. Density: 10.28 g/cm 3. The order of filling the orbitals with electrons in the Mo atom is an exception to the rule. Expected electronic configuration 1s 2 2s 2 2p 6 3s …
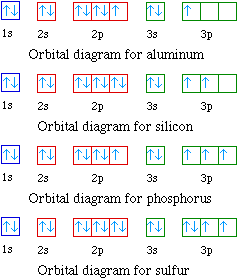
Silicon Orbital diagram, Electron configuration, and ... The Silicon orbital diagram contains 2 electrons in 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, the two electrons in the 3s orbital, and the remaining two electrons in the 3p orbital. The orbital diagram for a ground-state electron configuration of a Silicon atom is shown below-
S-Block Elements on the Periodic Table: Properties ... 23.09.2021 · The s orbital is spherical and can be occupied by a maximum of two electrons. Elements in column 1 have one electron in the s orbital, and elements in column 2 (plus helium) have two electrons in ...
Electron Configuration for Phosphorus (P) - UMD In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the ...
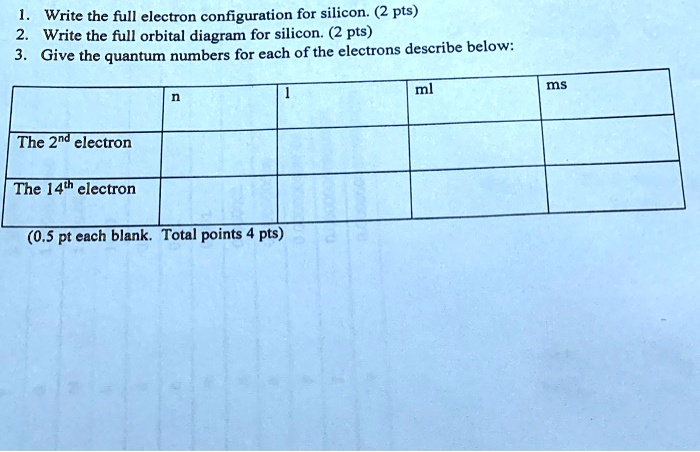
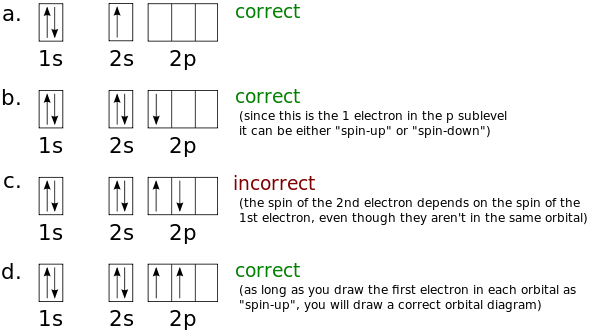
Electron Configurations and Orbital Box Diagrams ... An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin-one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the first 20 elements in the figure below.
Sodium Bohr Model - How to draw Bohr diagram for Sodium(Na) atom Electron dot diagram of a Sodium atom. The electron dot diagram also called lewis’s structure which represents the valence electrons of atoms. As, from the Bohr diagram of Sodium, we got to know, it has only 1 valence electron. So, just represent the 1 valence electrons around the Sodium atom as a dot.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
Silicon Bohr Model - How to draw Bohr diagram for Silicon ... Steps to draw the Bohr Model of Silicon atom 1. Find the number of protons, electrons, and neutrons in the Silicon atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Electrons are the negatively charged particles that orbit the nucleus of an atom
Nuclear Spin Quantum Memory in Silicon Carbide 21.04.2022 · Nuclear Spin Quantum Memory in Silicon Carbide ... tal potential splits the D-shell into one orbital singlet and two orbital doublets. Due to the spin-orbit inter- action the electronic structure for the active electron is given by ve Kramers doublets (KDs), which are pairs of states related to each other by time inversion. We show the nearest neighbor structure of the defect in …
Dangling bond - Wikipedia In chemistry, a dangling bond is an unsatisfied valence on an immobilized atom.An atom with a dangling bond is also referred to as an immobilized free radical or an immobilized radical, a reference to its structural and chemical similarity to a free radical.. When speaking of a dangling bond, one is generally referring to the state described above, containing one electron and thus …
What is the orbital diagram for Silicon? - Answers An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in ...
Lithium Bohr Model - How to draw Bohr diagram for Lithium ... Electron dot diagram of the Lithium atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Lithium, we got to know, it has 1 valence electron. So, just represent the 1 valence electrons around the Lithium atom as a dot.
Silicon Electron Configuration | Orbital Diagram For ... Silicon Orbital Diagram Well, the orbital diagram of Electron Configuration is the best source of information if you are willing to study the chemical bonding of silicon. This is a kind of qualitative diagram that explains the whole combining process of the element in the best possible manner.
How to Write the Orbital Diagram for Silicon (Si) - YouTube To write the orbital diagram for the Silicon atom (Si) first we need to write the electron configuration for just Si. To do that we need to find the number ...
Assymetric Induction - Michigan State University The following diagram shows a typical reaction of this kind, in which the chelating ligand is Y and the proposed transition state is enclosed in square brackets. The nucleophile is shown attacking the carbonyl group preferentially from the side of the smallest remaining substituent. Nine examples are listed in the table beneath the equation and two more are shown in the blue …
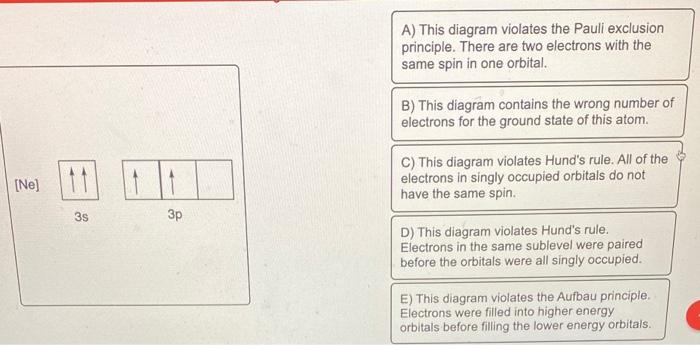
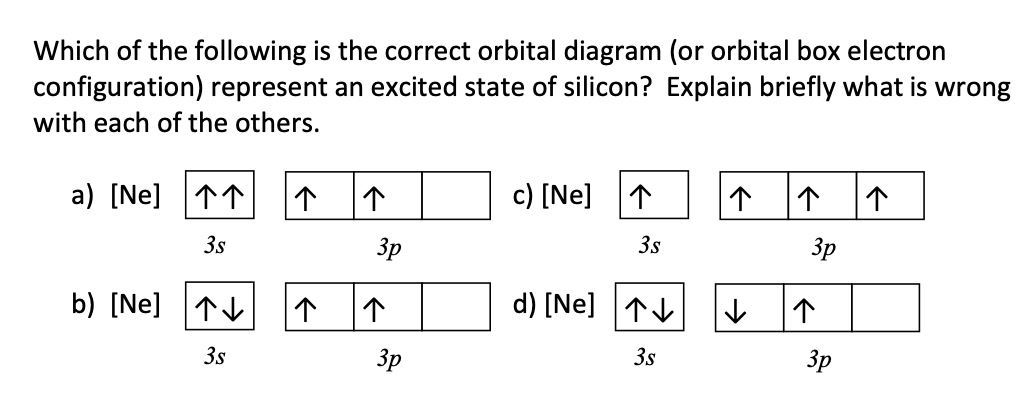
Solved Which of the following is the correct orbital ... Solved Which of the following is the correct orbital diagram | Chegg.com. Science. Chemistry. Chemistry questions and answers. Which of the following is the correct orbital diagram for silicon? 0 1s 2s 2p 3s 3p น น ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ i』2s 2p 3s 3p O Type here to search.
Silicon Electron Configuration (Si) with Orbital Diagram Silicon Orbital Diagram Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form. That is one box contains 2 electrons. And for silicon, there will be 7 box representations for 14 electrons in a pair.
Silicon(Si) electron configuration and orbital diagram Orbital diagram for silicon (Si) Silicon (Si) excited state electron configuration Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of silicon is 1s2 2s2 2p6 3s2 3p2. The valency of the element is determined by electron configuration in the excited state.
Bismuth(Bi) electron configuration and orbital diagram The 4f orbital is now full of electrons. So, the next ten electrons will enter the 5d orbital and the remaining three electrons will enter the 6p orbital. Therefore, the bismuth(Bi) electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10 6s 2 6p 3. How to write the orbital diagram for bismuth(Bi)?
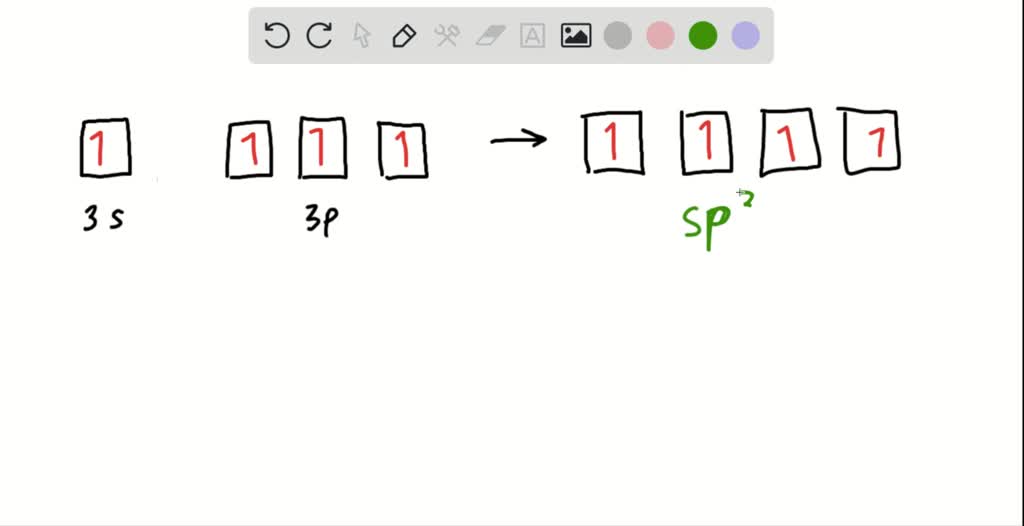
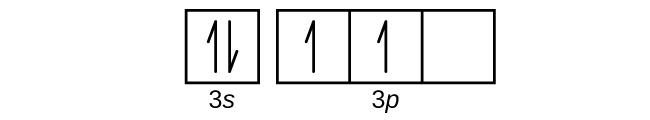
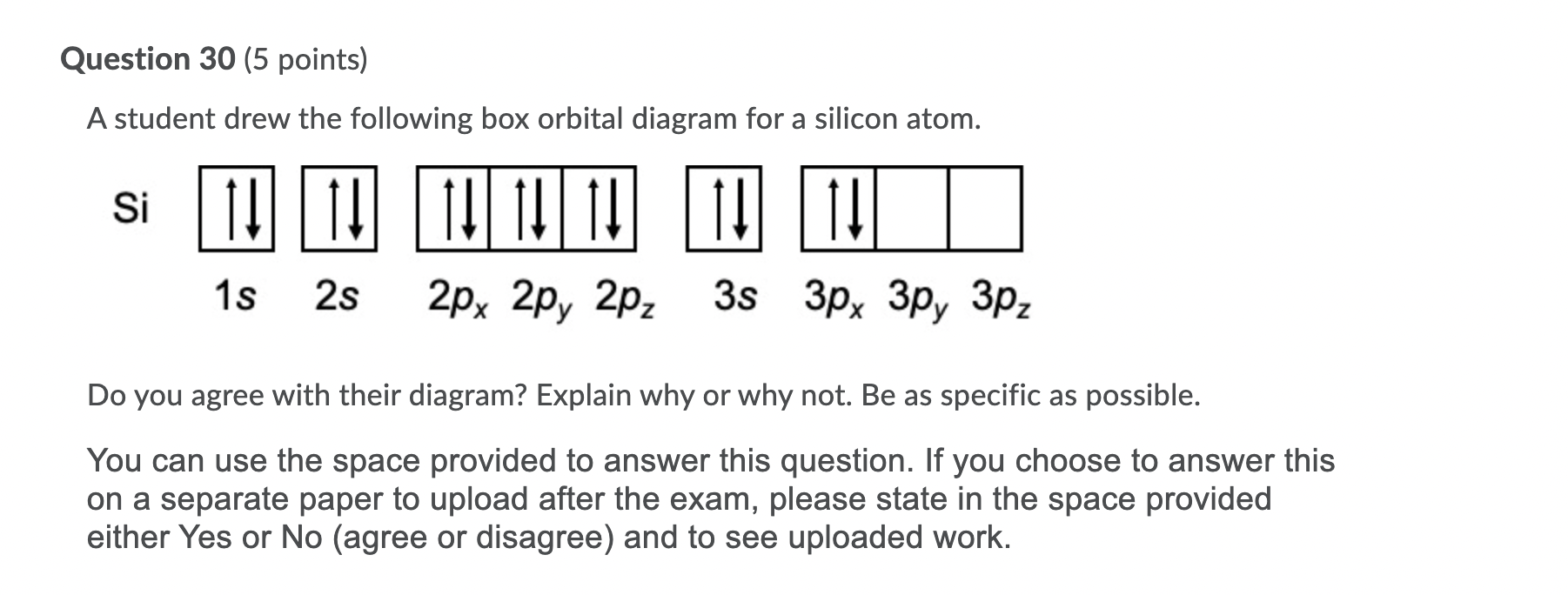





















0 Response to "40 orbital diagram for silicon"
Post a Comment