36 he2 2+ molecular orbital diagram
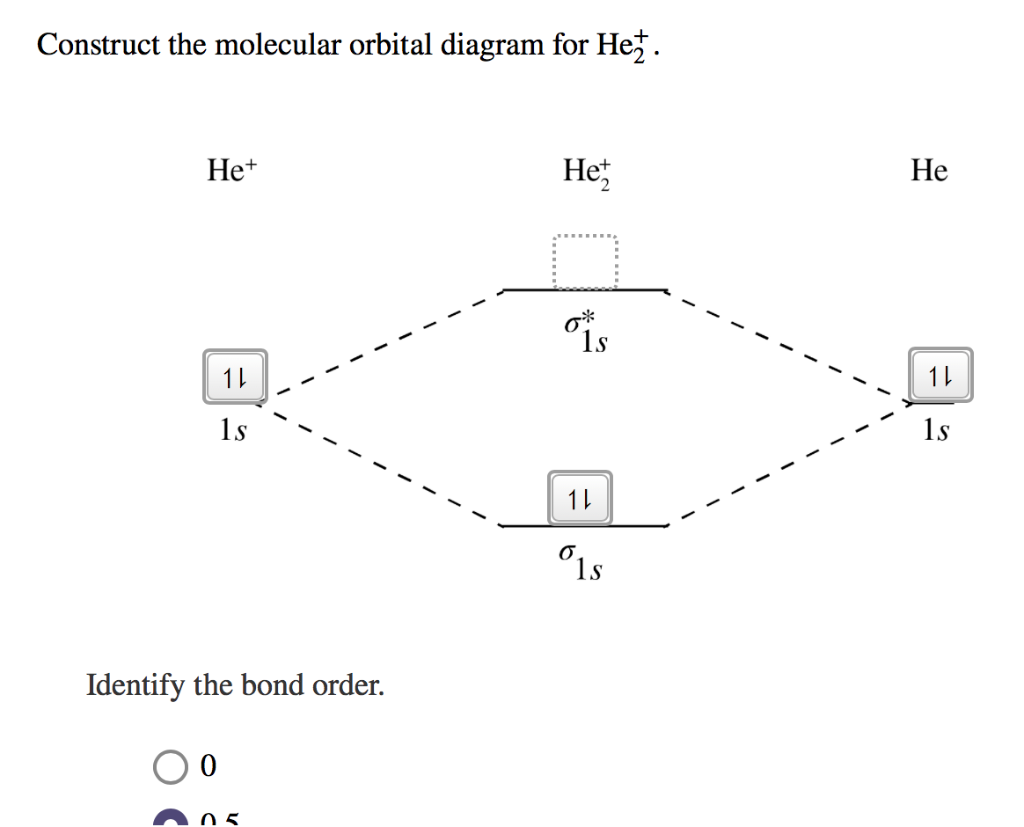
Solved Construct the molecular orbital diagram for | Chegg.com Answer : Number of electron : He = 2 He+ = 1 He cation dona …. View the full answer. Transcribed image text: Construct the molecular orbital diagram for Hez. Answer Bank 11 11 Energy 1s 11 1s 11 Atom Molecule Atom Het Het He Incorrect Identify the bond order. 0 0.5 OOOO 1.5 2 Incorrect. Previous question Next question. OneClass: construct the molecular orbital diagram for He2 ... construct the molecular orbital diagram for He2+2 and Sapling Learning Map d mcanoe Construct the molecular orbital diagram for Hez and then identify the bond order Bond order: 1s 1s D0.5 Ï . 1s 0 1.5 Atom Molecule Atom HeHe He Click within the blue boxes to add electrons O Next, Exit- Show full question Answer + 20 Watch
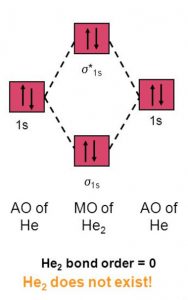
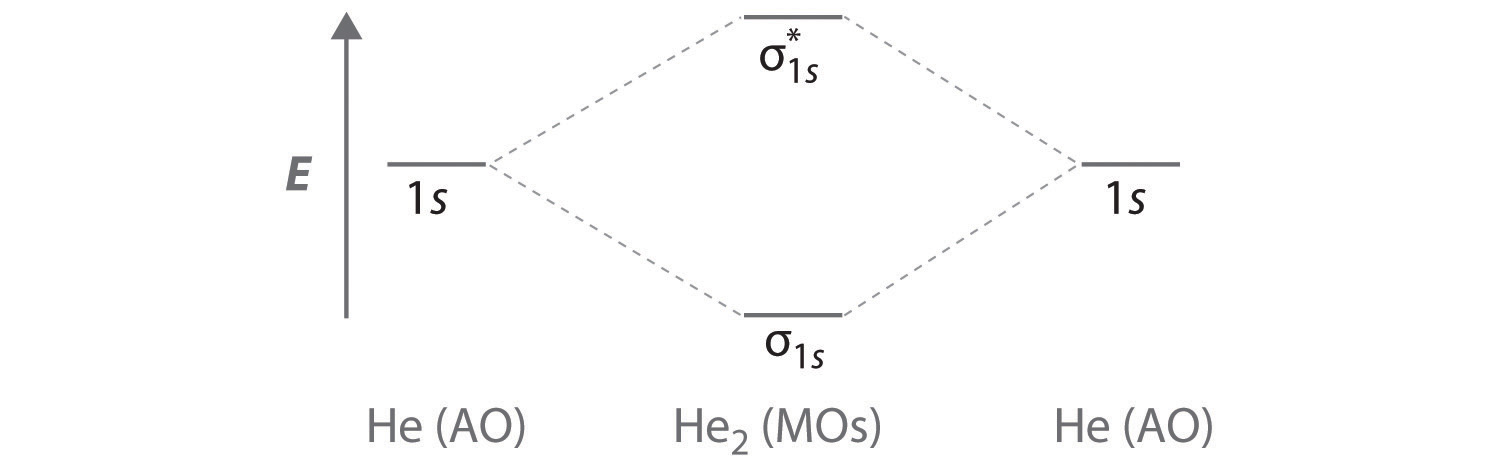
Do He2, He2(+), He2(2+) exist, stable? (Molecular Orbital ... In He2 (dihelium), the two 1s atomic orbitals overlap to create two molecular orbitals: sigma(1s) and sigma(1s)*. You fill these molecular orbitals with the...
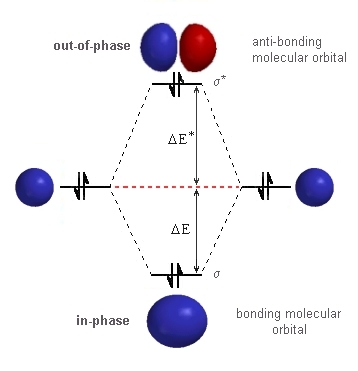
He2 2+ molecular orbital diagram
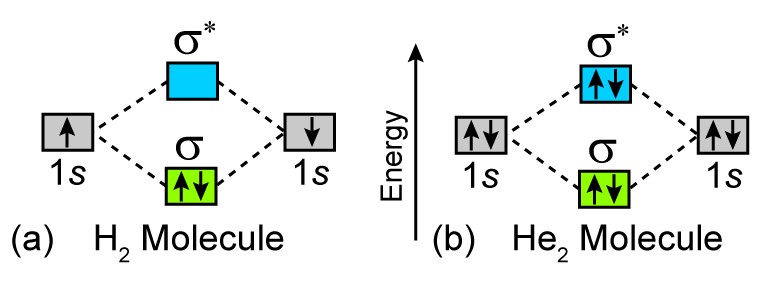
Why He2 molecule does not exist? Explain by MOT. Molecular Orbital Diagram for He 2 is (Refer to Image) Bond order= 2(No. of electrons in bonding molecular orbital)- (No. of electrons in anti-bonding Molecular orbital) = 22−2 =0 ∴He 2 bond order is 0. There is no bond existing between atoms of He 2 . So He 2 does not exist. Solve any question of Chemical Bonding and Molecular Structure with:- chemical bonding - Molecular orbitals of H2 and He2 ... The molecular orbital energy-level diagram shown in Figure 13 also applies (with changes of detail in the energies of the molecular orbitals) to the hypothetical species He 2. However, this species has four valence electrons, and its configuration would be 1σ 2 2σ 2. How do I calculate the bond order for H2- and H2+? | Socratic Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
He2 2+ molecular orbital diagram. Molecular Orbital Diagram of H2, He2, Li2 and Be2 ... 0:15 Molecular Orbital Diagram of Hydrogen Molecule1:39 Molecular Orbital Diagram of Helium Molecule2:54 Molecular Orbital Diagram of Lithium Molecule4:00 Mo... Answered: Is it possible for a He2 molecule to… | bartleby Solution for Is it possible for a He2 molecule to form? Explain with the use of a molecular orbital diagram and the bond order. close. Start your trial now! First week only $4.99! arrow_forward. learn. write. tutor. study resourcesexpand_more. Study Resources. We've got the study and writing resources you need for your assignments. ... __FULL__ Mo Diagram For He2 on uscomporthu Tara Seattle on __FULL__ Mo Diagram For He2. 8d69782dd3 Oct 6, 2014 — MO diagram of H2: In the case of H2, both electrons are in the σ1s orbital. MO diagram of He2: Electron configuration of He2: There is in.. Why He2 is not in molecular form? - Quora Answer (1 of 5): Helium(z=2) atom has 2 electrons in its outermost shell. So He2 molecule will have 2*2=4 electrons. According to Molecular Orbital theory ,the atomic orbitals of the two He atoms will combine to form 2 molecular orbitals(1 bonding and 1 anti-bonding). So Electronic Configuration...
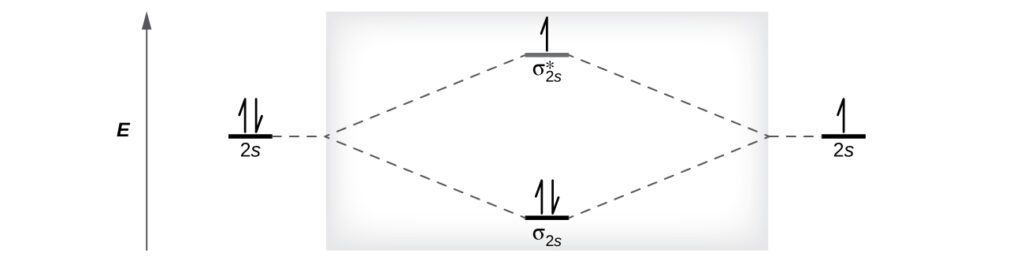
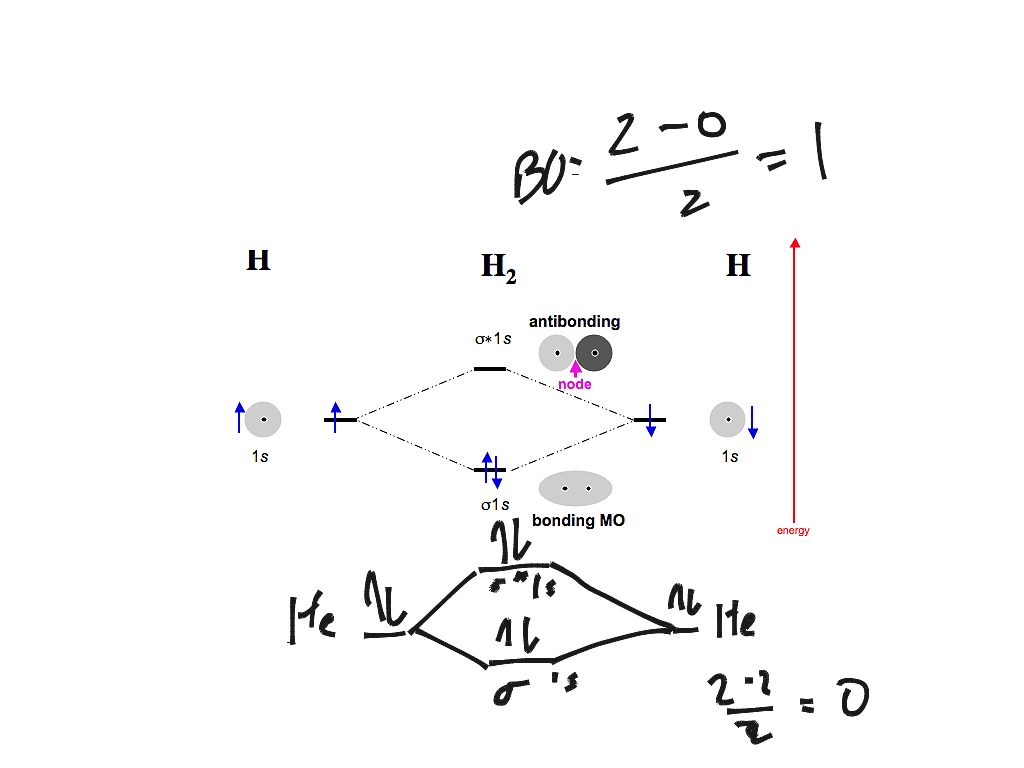
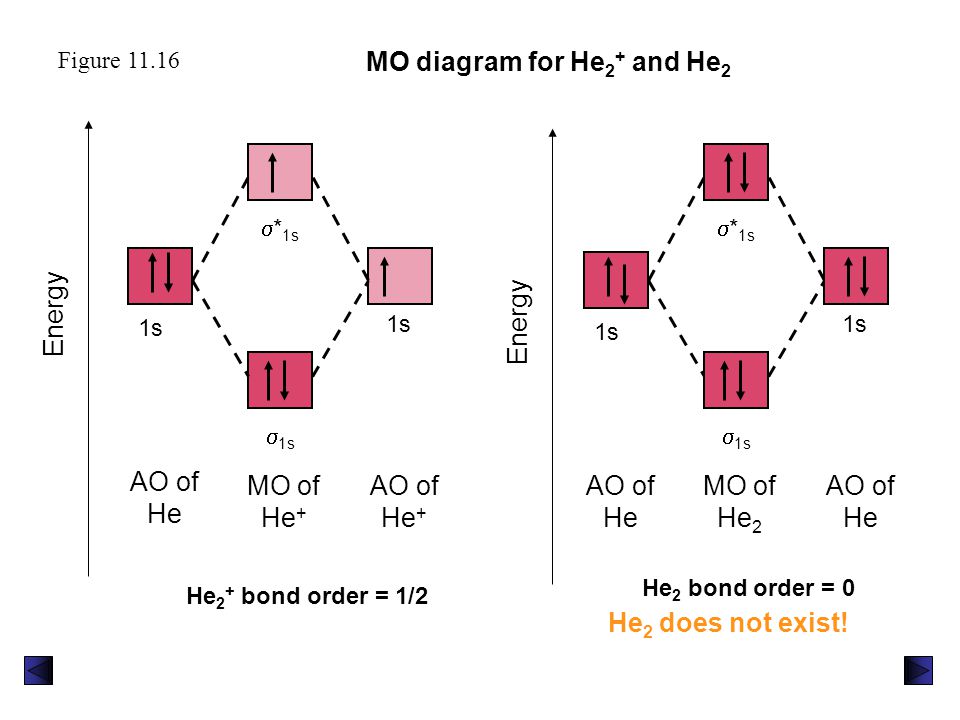
What is the molecular electronic configuration of ... - Quora Answer (1 of 3): Molecular electronic configuration for H2- is 1s2, 1s1*. Here s is used to represent the sigma symbol. What is the MOED of He2 molecule class 11 chemistry CBSE And we got from the diagram that Helium has two electrons in its bonding molecular orbital and two electrons in its anti-bonding molecular orbital. So its bond order will be: B o n d o r d e r = 1 2 [ 2 − 2] = 0. So, the bond comes out to be zero, therefore the H e 2 molecule is unstable and does not exist. 5.2.3: Diatomic Molecules of the First and Second Periods ... Figure 5.2.3. 1: Molecular Orbital Energy-Level Diagrams for the Diatomic Molecules of the Period 2 Elements. Unlike earlier diagrams, only the valence molecular orbital energy levels for the molecules are shown here (atomic orbitals not shown for simplicity). For Li2 through N2, the σ g ( 2 p) orbital is higher in energy than the π u ( 2 p ... He2 2+ Molecular Orbital Diagram - schematron.org (a) The H 2 + ion. A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.
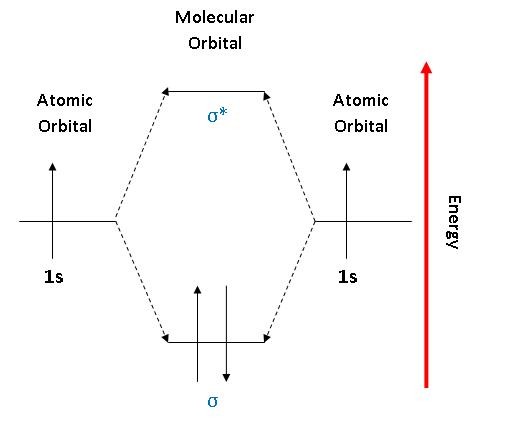
PDF 26. Correlation Diagrams: H and He Molecular Orbitals 2 Molecular Orbitals Below is the correlation diagram for two hydrogen atoms and the resulting H 2 molecule. Each atom has one electron before bonding. When the two hydrogen atoms bond, those two electrons occupy one of the two molecular orbitals that were created. 1) How does the energy of the two electrons in the H 2 He2 2+ Molecular Orbital Diagram - Wiring Diagrams He2 2+ Molecular Orbital Diagram The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H2 molecule is shown in Figure Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. Ochem: Bond order for He2 + and He2 2- : chemhelp If you continue filling up the homonuclear diatomic MO diagram, with neutral He2 you have 4 electrons which are in the 1sigma_g and 1sigma_u* bonding and anti-bonding orbitals respectively. The next two electrons should each go in the 2sigma_g bonding orbital, and should each contribute +0.5 to the bond order. (0.5 for a, 1 for d). Molecular Orbital Diagram For He2 A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
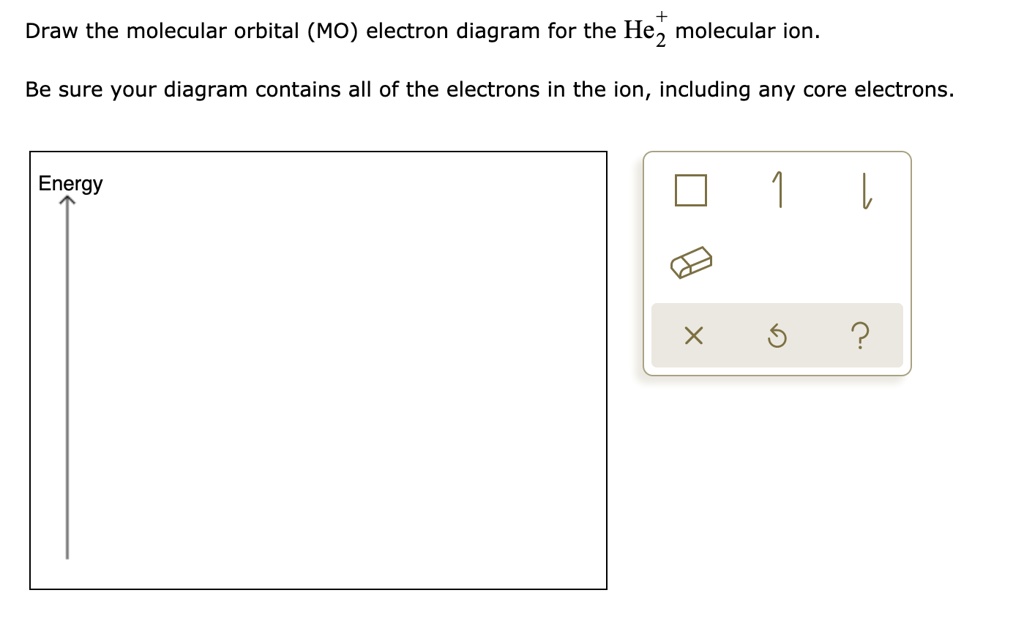
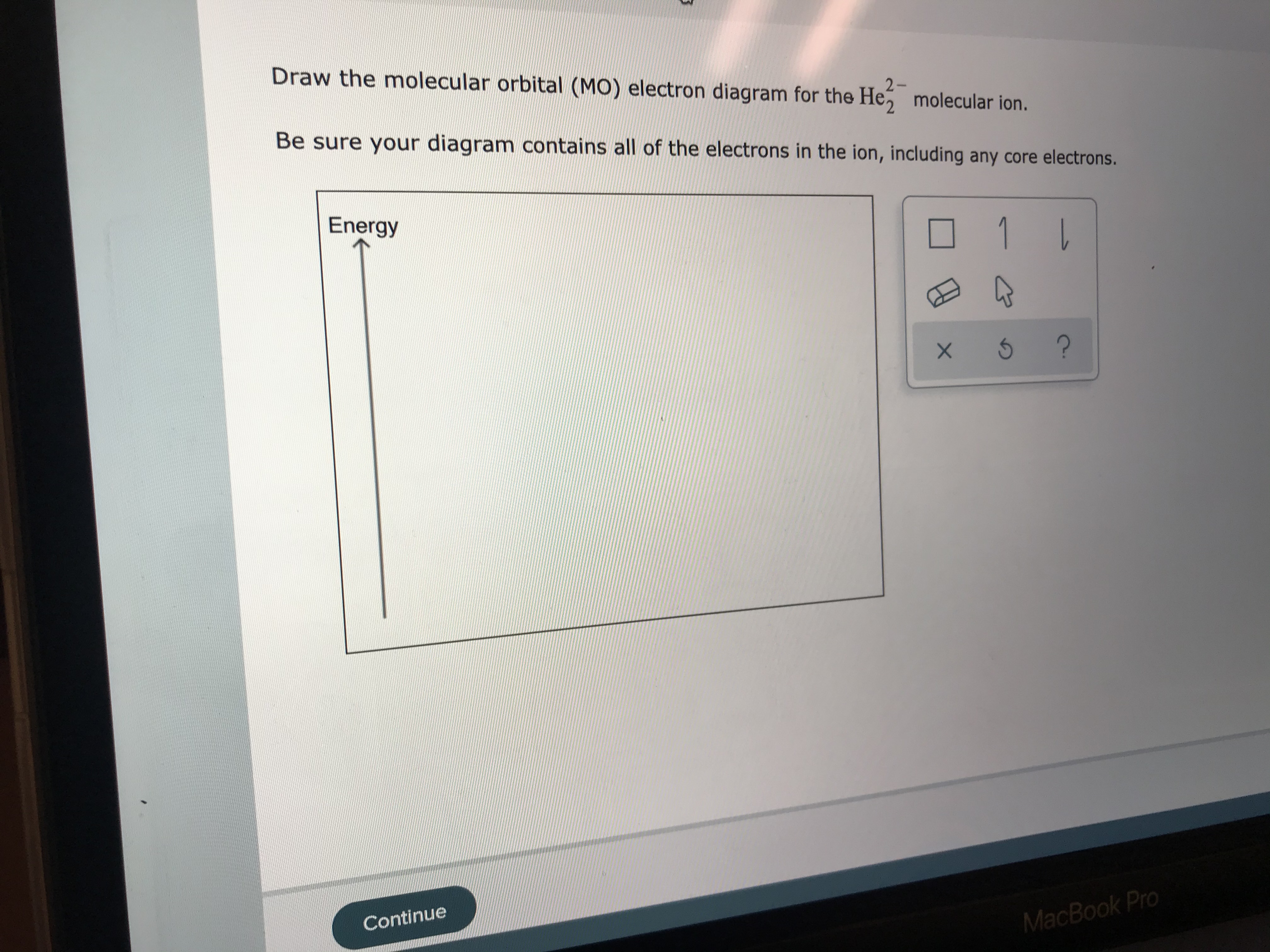
Solved Draw the molecular orbital (MO) electron diagram ... Draw the molecular orbital (MO) electron diagram for the He2^2- molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core electrons. Question: Draw the molecular orbital (MO) electron diagram for the He2^2- molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core ...
Construct The Molecular Orbital Diagram For He2 And Then ... Problem Construct the molecular orbital diagram for He 2 + and then identify the bond order. Since both molecular ions have a bond order of 1/2, they are approximately equally stable. Problem: Surprisingly, the hybridization of the starred oxygen in the following molecule is sp 2, not sp 3.
Molecular Orbital Diagram For He2 2+ - Wiring Diagrams Please note the diagram is for He2+ but the He-H is very similar answered Mar 21 '13 at A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.
Why is He2 not a stable molecule? - CHEMISTRY COMMUNITY Postby Chem_Mod » Wed Oct 26, 2016 6:31 am To answer the question, you must construct a molecular orbital (MO) diagram for the hypothetical He 2 molecule. The σ 1s bonding and antibonding orbitals will be full. Calculating the bond order results in 0. In other words, no bond can be sustained between two He atoms according to MO theory. Top
Molecular Orbital Diagram For He2+ - schematron.org The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = .
How do I calculate the bond order for H2- and H2+? | Socratic Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
chemical bonding - Molecular orbitals of H2 and He2 ... The molecular orbital energy-level diagram shown in Figure 13 also applies (with changes of detail in the energies of the molecular orbitals) to the hypothetical species He 2. However, this species has four valence electrons, and its configuration would be 1σ 2 2σ 2.
Why He2 molecule does not exist? Explain by MOT. Molecular Orbital Diagram for He 2 is (Refer to Image) Bond order= 2(No. of electrons in bonding molecular orbital)- (No. of electrons in anti-bonding Molecular orbital) = 22−2 =0 ∴He 2 bond order is 0. There is no bond existing between atoms of He 2 . So He 2 does not exist. Solve any question of Chemical Bonding and Molecular Structure with:-



















0 Response to "36 he2 2+ molecular orbital diagram"
Post a Comment